|
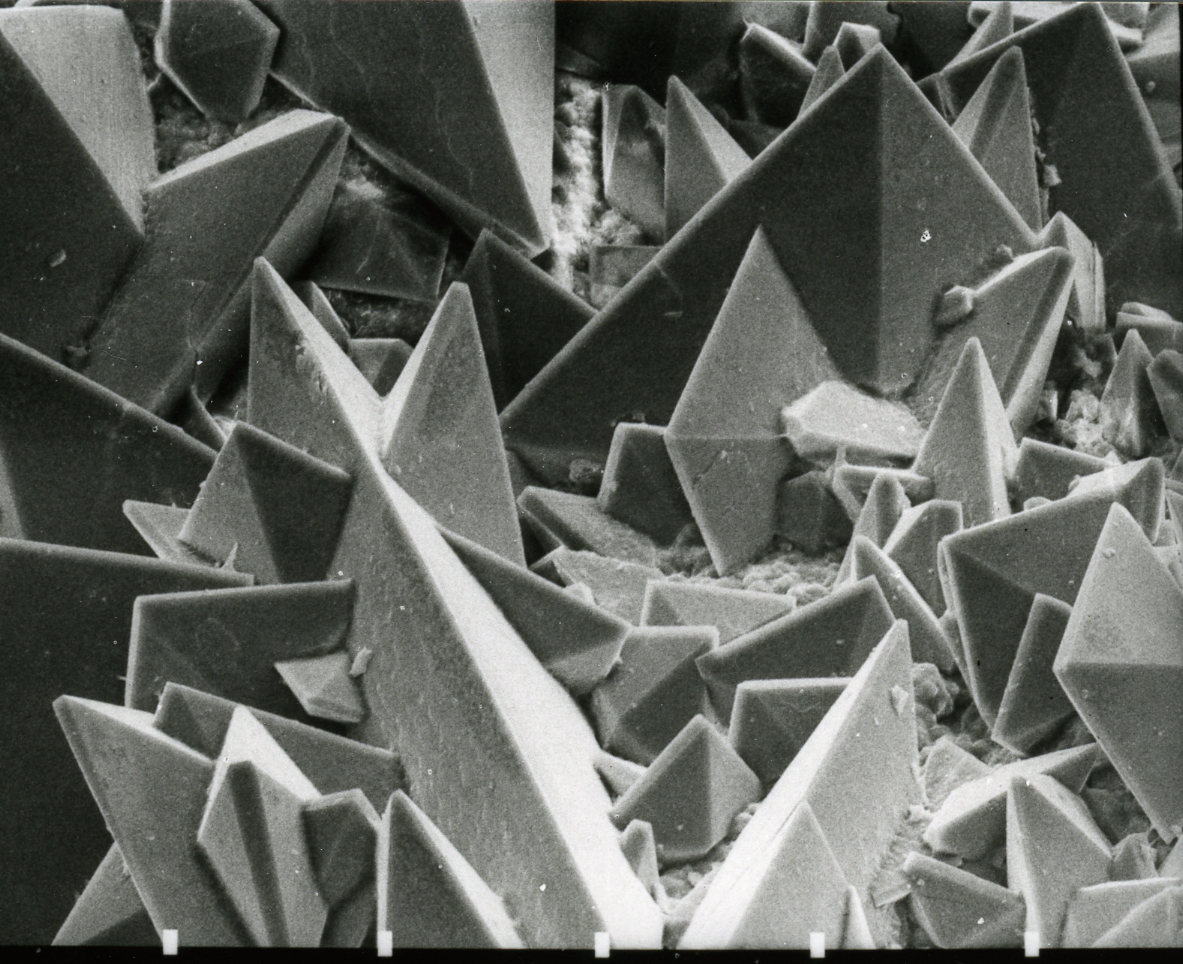
Sodium Oxalate
Sodium oxalate, or disodium oxalate, is the sodium salt of oxalic acid with the formula Na2C2O4. It is a white, crystalline, odorless solid, that decomposes above 290 °C. Disodium oxalate can act as a reducing agent, and it may be used as a primary standard for standardizing potassium permanganate (KMnO4) solutions. The mineral form of sodium oxalate is natroxalate. It is only very rarely found and restricted to extremely sodic conditions of ultra-alkaline pegmatites. Preparation Sodium oxalate can be prepared through the neutralization of oxalic acid with sodium hydroxide (NaOH) in a 1:2 acid-to-base molar ratio. Evaporation yields the anhydrous oxalateH. W. Foote and John E. Vance (1933), "The system; sodium iodate, sodium oxalate, water". ''American Journal of Science'', series 5, volume 26, issue 151, pages 16-18. that can be thoroughly dried by heating to between 200 and 250 °C. Half-neutralization can be accomplished with NaOH in a 1:1 ratio which produces NaHC2O4, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Hydrogenoxalate
Sodium hydrogenoxalate is salt (ionic compound) of formula , consisting of sodium cation An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...s and hydrogenoxalate anions or . The anion can be described as the result of removing one hydrogen ion from oxalic acid , or adding one to the oxalate anion . Properties Hydrates The compound is commonly encountered as the hydrate, anhydrous form or as the monohydrate ·. Both are colorless crystalline solids at ambient temperature. The monohydrate can be obtained by evaporating a solution of the compound at room temperature.C. Ramki, R. Ezhil Vizhi (2017): "Growth, optical, electrical and mechanical properties of sodium hydrogen oxalate hydrate (NaHC2O4·H2O) single crystal for NLO applications". ''Materials Chemistry and Physics'', volu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Formate
Sodium formate, HCOONa, is the sodium salt of formic acid, HCOOH. It usually appears as a white deliquescent powder. Preparation For commercial use, sodium formate is produced by absorbing carbon monoxide under pressure in solid sodium hydroxide at 130 °C and 6-8 bar pressure:Arnold Willmes, ''Taschenbuch Chemische Substanzen'', Harri Deutsch, Frankfurt (M.), 2007. :CO + NaOH → HCO2Na Because of the low-cost and large-scale availability of formic acid by carbonylation of methanol and hydrolysis of the resulting methyl formate, sodium formate is usually prepared by neutralizing formic acid with sodium hydroxide. Sodium formate is also unavoidably formed as a by-product in the final step of the pentaerythritol synthesis and in the crossed Cannizzaro reaction of formaldehyde with the aldol reaction product trimethylol acetaldehyde -hydroxy-2,2-bis(hydroxymethyl)propanal In the laboratory, sodium formate can be prepared by neutralizing formic acid with sodium carbonat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Oxalate
Calcium oxalate (in archaic terminology, oxalate of lime) is a calcium salt of oxalic acid with the chemical formula . It forms hydrates , where ''n'' varies from 1 to 3. Anhydrous and all hydrated forms are colorless or white. The monohydrate occurs naturally as the mineral whewellite, forming envelope-shaped crystals, known in plants as raphides. The two rarer hydrates are dihydrate , which occurs naturally as the mineral weddellite, and trihydrate , which occurs naturally as the mineral caoxite, are also recognized. Some foods have high quantities of calcium oxalates and can produce sores and numbing on ingestion and may even be fatal. Tribes with diets that depend highly on fruits and vegetables high in calcium oxalate, such as in Micronesia, reduce the level of it by boiling and cooking them. They are a constituent in 76% of human kidney stones. Calcium oxalate is also found in beerstone, a scale that forms on containers used in breweries. Occurrence Many plants accumu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Citrate
Citric acid is an organic compound with the chemical formula HOC(CO2H)(CH2CO2H)2. It is a colorless weak organic acid. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in the metabolism of all aerobic organisms. More than two million tons of citric acid are manufactured every year. It is used widely as an acidifier, as a flavoring, and a chelating agent. A citrate is a derivative of citric acid; that is, the salts, esters, and the polyatomic anion found in solution. An example of the former, a salt is trisodium citrate; an ester is triethyl citrate. When part of a salt, the formula of the citrate anion is written as or . Natural occurrence and industrial production Citric acid occurs in a variety of fruits and vegetables, most notably citrus fruits. Lemons and limes have particularly high concentrations of the acid; it can constitute as much as 8% of the dry weight of these fruits (about 47 g/L in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MSDS
A safety data sheet (SDS), material safety data sheet (MSDS), or product safety data sheet (PSDS) is a document that lists information relating to occupational safety and health for the use of various substances and products. SDSs are a widely used system for cataloguing information on chemicals, chemical compounds, and chemical mixtures. SDS information may include instructions for the safe use and potential hazards associated with a particular material or product, along with spill-handling procedures. The older MSDS formats could vary from source to source within a country depending on national requirements; however, the newer SDS format is internationally standardized. An SDS for a substance is not primarily intended for use by the general consumer, focusing instead on the hazards of working with the material in an occupational setting. There is also a duty to properly label substances on the basis of physico-chemical, health, or environmental risk. Labels can include hazard ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxalate
Oxalate (IUPAC: ethanedioate) is an anion with the formula C2O42−. This dianion is colorless. It occurs naturally, including in some foods. It forms a variety of salts, for example sodium oxalate (Na2C2O4), and several esters such as dimethyl oxalate (C2O4(CH3)2). It is a conjugate base of oxalic acid. At neutral pH in aqueous solution, oxalic acid converts completely to oxalate. Relationship to oxalic acid The dissociation of protons from oxalic acid proceeds in a stepwise manner; as for other polyprotic acids, loss of a single proton results in the monovalent hydrogenoxalate anion . A salt with this anion is sometimes called an acid oxalate, monobasic oxalate, or hydrogen oxalate. The equilibrium constant ( ''K''a) for loss of the first proton is (p''K''a = 1.27). The loss of the second proton, which yields the oxalate ion, has an equilibrium constant of (p''K''a = 4.28). These values imply, in solutions with neutral pH, no oxalic acid and only trace am ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Dioxide
Carbon dioxide (chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transparent to visible light but absorbs infrared radiation, acting as a greenhouse gas. It is a trace gas in Earth's atmosphere at 421 parts per million (ppm), or about 0.04% by volume (as of May 2022), having risen from pre-industrial levels of 280 ppm. Burning fossil fuels is the primary cause of these increased CO2 concentrations and also the primary cause of climate change.IPCC (2022Summary for policy makersiClimate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA Carbon dioxide is soluble in water and is found in groundwater, lakes, ice caps, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Vanadium Oxibronze
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable isotope is 23Na. The free metal does not occur in nature, and must be prepared from compounds. Sodium is the sixth most abundant element in the Earth's crust and exists in numerous minerals such as feldspars, sodalite, and halite (NaCl). Many salts of sodium are highly water-soluble: sodium ions have been leached by the action of water from the Earth's minerals over eons, and thus sodium and chlorine are the most common dissolved elements by weight in the oceans. Sodium was first isolated by Humphry Davy in 1807 by the electrolysis of sodium hydroxide. Among many other useful sodium compounds, sodium hydroxide ( lye) is used in soap manufacture, and sodium chloride ( edible salt) is a de-icing agent and a nutrient for animals ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vanadium Pentoxide
Vanadium(V) oxide (''vanadia'') is the inorganic compound with the formula V2 O5. Commonly known as vanadium pentoxide, it is a brown/yellow solid, although when freshly precipitated from aqueous solution, its colour is deep orange. Because of its high oxidation state, it is both an amphoteric oxide and an oxidizing agent. From the industrial perspective, it is the most important compound of vanadium, being the principal precursor to alloys of vanadium and is a widely used industrial catalyst. The mineral form of this compound, shcherbinaite, is extremely rare, almost always found among fumaroles. A mineral trihydrate, V2O5·3H2O, is also known under the name of navajoite. Chemical properties Reduction to lower oxides Upon heating a mixture of vanadium(V) oxide and vanadium(III) oxide, comproportionation occurs to give vanadium(IV) oxide, as a deep-blue solid: :V2O5 + V2O3 → 4 VO2 The reduction can also be effected by oxalic acid, carbon monoxide, and sulfur dioxide. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Monoxide
Carbon monoxide (chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simplest molecule of the oxocarbon family. In coordination complexes the carbon monoxide ligand is called carbonyl. It is a key ingredient in many processes in industrial chemistry. The most common source of carbon monoxide is the partial combustion of carbon-containing compounds, when insufficient oxygen or heat is present to produce carbon dioxide. There are also numerous environmental and biological sources that generate and emit a significant amount of carbon monoxide. It is important in the production of many compounds, including drugs, fragrances, and fuels. Upon emission into the atmosphere, carbon monoxide affects several processes that contribute to climate change. Carbon monoxide has important biological roles across phylogenetic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Carbonate
Sodium carbonate, , (also known as washing soda, soda ash and soda crystals) is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield moderately alkaline solutions in water. Historically, it was extracted from the ashes of plants growing in sodium-rich soils. Because the ashes of these sodium-rich plants were noticeably different from ashes of wood (once used to produce potash), sodium carbonate became known as "soda ash". It is produced in large quantities from sodium chloride and limestone by the Solvay process. Hydrates Sodium carbonate is obtained as three hydrates and as the anhydrous salt: * sodium carbonate decahydrate (natron), Na2CO3·10H2O, which readily efflorescence, effloresces to form the monohydrate. * sodium carbonate heptahydrate (not known in mineral form), Na2CO3·7H2O. * sodium carbonate monohydrate (thermonatrite), Na2CO3·H2O. Also known as crystal carbonate. * anhydrous sodium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |