|
Slit (gene)
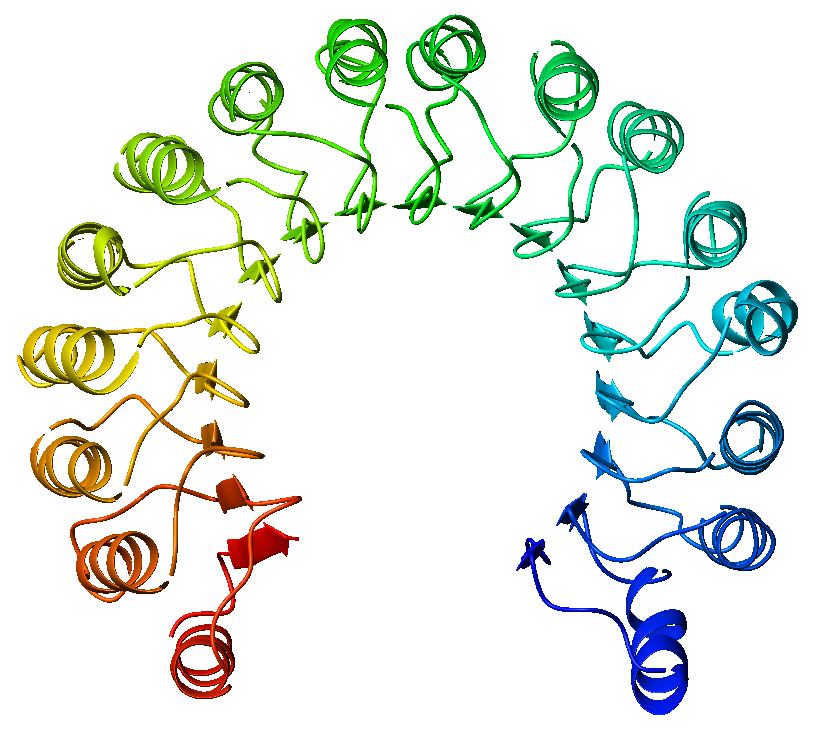
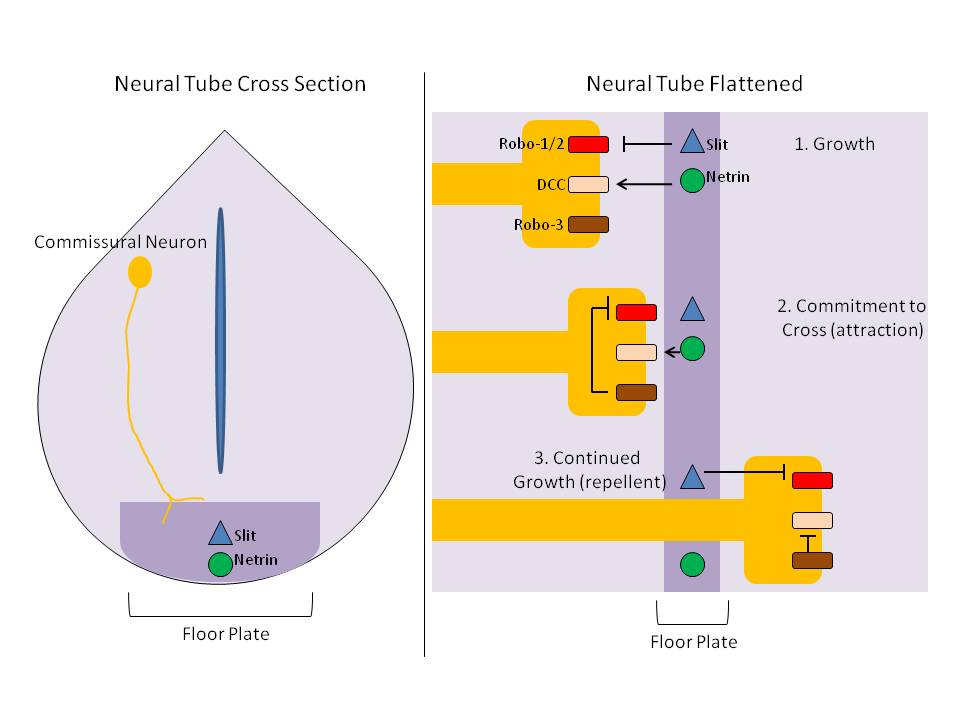
Slit is a protein family, family of secreted extracellular matrix proteins which play an important signalling role in the neural development of most bilaterians (animals with bilateral symmetry). While lower animal species, including insects and nematode worms, possess a single Slit gene, humans, mice and other vertebrates possess three Slit homologs: SLIT1, Slit1, SLIT2, Slit2 and SLIT3, Slit3. Human ''Slits'' have been shown to be involved in certain pathological conditions, such as cancer and inflammation. The ventral midline of the central nervous system is a key place where axons can either decide to cross and laterally project or stay on the same side of the brain. The main function of Slit proteins is to act as midline repellents, preventing the crossing of longitudinal axons through the midline of the central nervous system of most bilaterian animal species, including mouse, mice, chickens, humans, insects, nematode worms and planarians. It also prevents the recrossing ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SLIT1
Slit homolog 1 protein is a protein that in humans is encoded by the ''SLIT1'' gene In biology, the word gene (from , ; "... Wilhelm Johannsen coined the word gene to describe the Mendelian units of heredity..." meaning ''generation'' or ''birth'' or ''gender'') can have several different meanings. The Mendelian gene is a b .... References Further reading * * * * * * * * * * {{protein-stub Slit proteins ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Floor Plate
The floor plate is a structure integral to the developing nervous system of vertebrate organisms. Located on the ventral midline of the embryonic neural tube, the floor plate is a specialized glial structure that spans the anteroposterior axis from the midbrain to the tail regions. It has been shown that the floor plate is conserved among vertebrates, such as zebrafish and mice, with homologous structures in invertebrates such as the fruit fly ''Drosophila'' and the nematode ''C. elegans''. Functionally, the structure serves as an organizer to ventralize tissues in the embryo as well as to guide neuronal positioning and differentiation along the dorsoventral axis of the neural tube. Induction Induction of the floor plate during embryogenesis of vertebrate embryos has been studied extensively in chick and zebrafish and occurs as a result of a complex signaling network among tissues, the details of which have yet to be fully refined. Currently there are several competing lines o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caenorhabditis Elegans
''Caenorhabditis elegans'' () is a free-living transparent nematode about 1 mm in length that lives in temperate soil environments. It is the type species of its genus. The name is a blend of the Greek ''caeno-'' (recent), ''rhabditis'' (rod-like) and Latin ''elegans'' (elegant). In 1900, Maupas initially named it '' Rhabditides elegans.'' Osche placed it in the subgenus ''Caenorhabditis'' in 1952, and in 1955, Dougherty raised ''Caenorhabditis'' to the status of genus. ''C. elegans'' is an unsegmented pseudocoelomate and lacks respiratory or circulatory systems. Most of these nematodes are hermaphrodites and a few are males. Males have specialised tails for mating that include spicules. In 1963, Sydney Brenner proposed research into ''C. elegans,'' primarily in the area of neuronal development. In 1974, he began research into the molecular and developmental biology of ''C. elegans'', which has since been extensively used as a model organism. It was the first mult ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drosophila
''Drosophila'' () is a genus of flies, belonging to the family Drosophilidae, whose members are often called "small fruit flies" or (less frequently) pomace flies, vinegar flies, or wine flies, a reference to the characteristic of many species to linger around overripe or rotting fruit. They should not be confused with the Tephritidae, a related family, which are also called fruit flies (sometimes referred to as "true fruit flies"); tephritids feed primarily on unripe or ripe fruit, with many species being regarded as destructive agricultural pests, especially the Mediterranean fruit fly. One species of ''Drosophila'' in particular, '' D. melanogaster'', has been heavily used in research in genetics and is a common model organism in developmental biology. The terms "fruit fly" and "''Drosophila''" are often used synonymously with ''D. melanogaster'' in modern biological literature. The entire genus, however, contains more than 1,500 species and is very diverse in appearan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protease
A protease (also called a peptidase, proteinase, or proteolytic enzyme) is an enzyme that catalyzes (increases reaction rate or "speeds up") proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the formation of new protein products. They do this by cleaving the peptide bonds within proteins by hydrolysis, a reaction where water breaks bonds. Proteases are involved in many biological functions, including digestion of ingested proteins, protein catabolism (breakdown of old proteins), and cell signaling. In the absence of functional accelerants, proteolysis would be very slow, taking hundreds of years. Proteases can be found in all forms of life and viruses. They have independently evolved multiple times, and different classes of protease can perform the same reaction by completely different catalytic mechanisms. Hierarchy of proteases Based on catalytic residue Proteases can be classified into seven broad groups: * Serine protea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cystine Knot
A cystine knot is a protein structural motif containing three disulfide bridges (formed from pairs of cysteine residues). The sections of polypeptide that occur between two of them form a loop through which a third disulfide bond passes, forming a rotaxane substructure. The cystine knot motif stabilizes protein structure and is conserved in proteins across various species. There are three types of cystine knot, which differ in the topology of the disulfide bonds: * The growth factor cystine knot (GFCK) * inhibitor cystine knot (ICK) common in spider and snail toxins * Cyclic Cystine Knot, or cyclotide The growth factor cystine knot was first observed in the structure of nerve growth factor (NGF), solved by X-ray crystallography and published in 1991 by Tom Blundell in Nature.; The GFCK is present in four superfamilies. These include nerve growth factor, transforming growth factor beta (TGF-β), platelet-derived growth factor, and glycoprotein hormones including human chorio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-terminus
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is translated from messenger RNA, it is created from N-terminus to C-terminus. The convention for writing peptide sequences is to put the C-terminal end on the right and write the sequence from N- to C-terminus. Chemistry Each amino acid has a carboxyl group and an amine group. Amino acids link to one another to form a chain by a dehydration reaction which joins the amine group of one amino acid to the carboxyl group of the next. Thus polypeptide chains have an end with an unbound carboxyl group, the C-terminus, and an end with an unbound amine group, the N-terminus. Proteins are naturally synthesized starting from the N-terminus and ending at the C-terminus. Function C-terminal retention signals While the N-terminus of a protein often ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
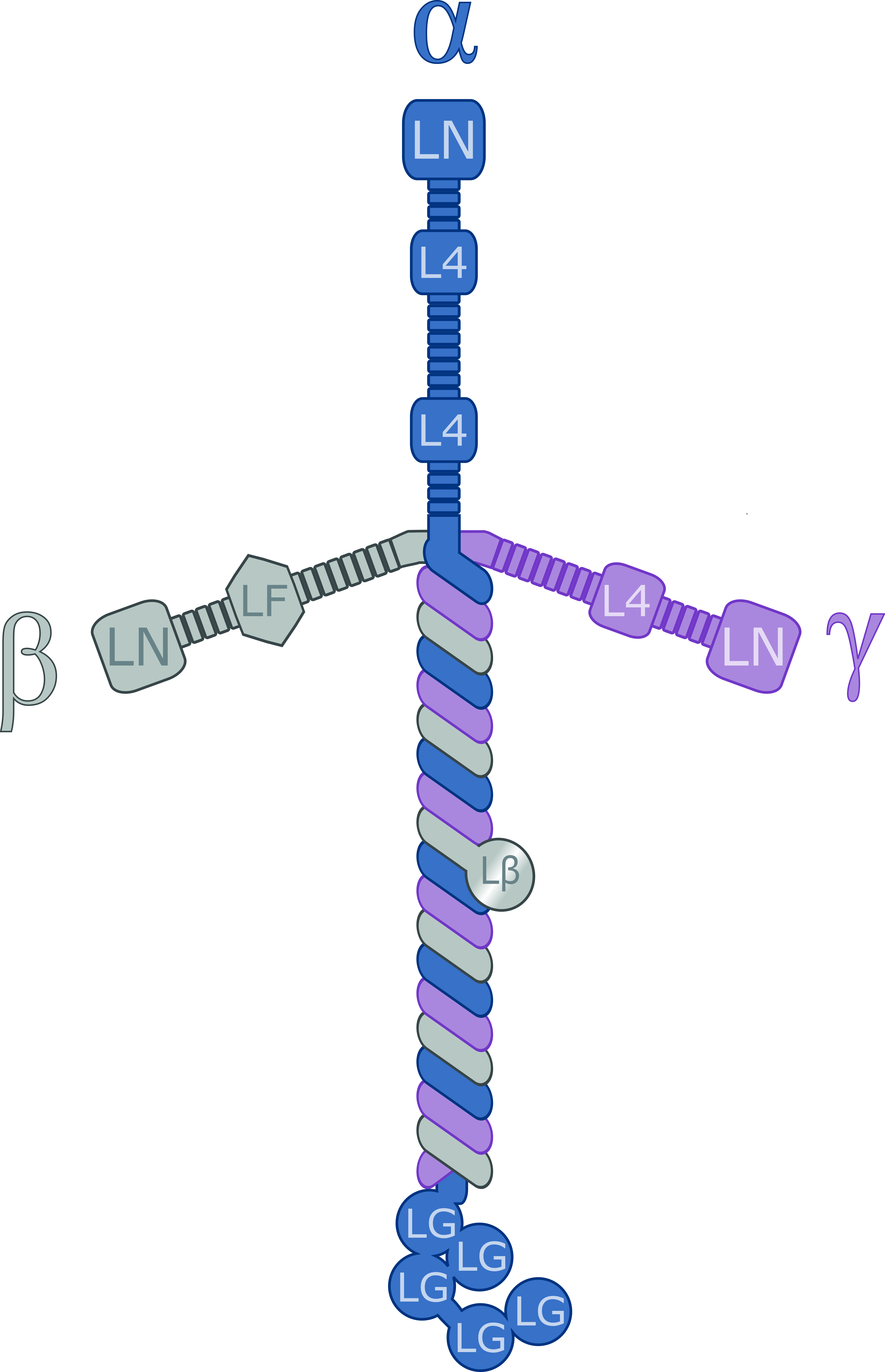
Laminin
Laminins are a family of glycoproteins of the extracellular matrix of all animals. They are major components of the basal lamina (one of the layers of the basement membrane), the protein network foundation for most cells and organs. The laminins are an important and biologically active part of the basal lamina, influencing cell differentiation, migration, and adhesion. Laminins are heterotrimeric proteins with a high molecular mass (~400 to ~900 kDa). They contain three different chains (α, β and γ) encoded by five, four, and three paralogous genes in humans, respectively. The laminin molecules are named according to their chain composition. Thus, laminin-511 contains α5, β1, and γ1 chains. Fourteen other chain combinations have been identified ''in vivo''. The trimeric proteins intersect to form a cross-like structure that can bind to other cell membrane and extracellular matrix molecules. The three shorter arms are particularly good at binding to other laminin molecules, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epidermal Growth Factor
Epidermal growth factor (EGF) is a protein that stimulates cell growth and differentiation by binding to its receptor, EGFR. Human EGF is 6-k Da and has 53 amino acid residues and three intramolecular disulfide bonds. EGF was originally described as a secreted peptide found in the submaxillary glands of mice and in human urine. EGF has since been found in many human tissues, including platelets, submandibular gland (submaxillary gland), and parotid gland. Initially, human EGF was known as urogastrone. Structure In humans, EGF has 53 amino acids (sequence NSDSECPLSHDGYCLHDGVCMYIEALDKYACNCVVGYIGERCQYRDLKWWELR), with a molecular mass of around 6 kDa. It contains three disulfide bridges (Cys6-Cys20, Cys14-Cys31, Cys33-Cys42). Function EGF, via binding to its cognate receptor, results in cellular proliferation, differentiation, and survival. Salivary EGF, which seems to be regulated by dietary inorganic iodine, also plays an important physiological role in the mai ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-terminus
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the amine group is bonded to the carboxylic group of another amino acid, making it a chain. That leaves a free carboxylic group at one end of the peptide, called the C-terminus, and a free amine group on the other end called the N-terminus. By convention, peptide sequences are written N-terminus to C-terminus, left to right (in LTR writing systems). This correlates the translation direction to the text direction, because when a protein is translated from messenger RNA, it is created from the N-terminus to the C-terminus, as amino acids are added to the carboxyl end of the protein. Chemistry Each amino acid has an amine group and a carboxylic group. Amino acids link to one another by peptide bonds which form through a dehydration reaction that ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leucine-rich Repeat
A leucine-rich repeat (LRR) is a protein structural motif that forms an α/β horseshoe fold. It is composed of repeating 20–30 amino acid stretches that are unusually rich in the hydrophobic amino acid leucine. These tandem repeats commonly fold together to form a solenoid protein domain, termed leucine-rich repeat domain. Typically, each repeat unit has beta strand- turn-alpha helix structure, and the assembled domain, composed of many such repeats, has a horseshoe shape with an interior parallel beta sheet and an exterior array of helices. One face of the beta sheet and one side of the helix array are exposed to solvent and are therefore dominated by hydrophilic residues. The region between the helices and sheets is the protein's hydrophobic core and is tightly sterically packed with leucine residues. Leucine-rich repeats are frequently involved in the formation of protein–protein interactions. Examples Leucine-rich repeat motifs have been identified in a large n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Growth Cones
Growth may refer to: Biology * Auxology, the study of all aspects of human physical growth * Bacterial growth * Cell growth * Growth hormone, a peptide hormone that stimulates growth * Human development (biology) * Plant growth * Secondary growth, growth that thickens woody plants Economics * Economic growth, the increase in the inflation-adjusted market value of the goods and services * Growth investing, a style of investment strategy focused on capital appreciation Mathematics * Exponential growth, also called geometric growth * Hyperbolic growth * Linear growth, refers to two distinct but related notions * Logistic growth, characterized as an S curve Social science * Developmental psychology * Erikson's stages of psychosocial development * Human development (humanity) * Personal development * Population growth Other uses * ''Growth'' (film), a 2010 American horror film * Izaugsme (''Growth''), a Latvian political party * ''Grown'' (album), by 2PM See also * Grow ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |