|
Saturated Spectroscopy
Saturated spectroscopy is the method by which the exact energy of the hyperfine transitions within an atom can be found. When a monochromatic light is shone through an atom, the absorption cross-section is broadened due to Doppler broadening. Saturated spectroscopy allows the doppler broadened peak to be resolved so that the exact transitions can be found. More than a decade after the first demonstration of spectral hole burning (or Lamb dip, a result of saturated absorption process) inside HeNe laser cavity at 1.1 μm in 1962, the greater majority of SA spectroscopy research was carried out with gas lasers and molecules in the mid-infrared. But because SA requires high laser intensity, and the gas molecules usually have widely spread strong absorption spectra only in the mid-IR, while compact widely tunable mid-IR lasers were slow to develop, the SA technique has not been widely used for molecular chemical analysis besides precision metrology, which only been limited to the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hyperfine Transitions
In atomic physics, hyperfine structure is defined by small shifts in otherwise degenerate energy levels and the resulting splittings in those energy levels of atoms, molecules, and ions, due to electromagnetic multipole interaction between the nucleus and electron clouds. In atoms, hyperfine structure arises from the energy of the nuclear magnetic dipole moment interacting with the magnetic field generated by the electrons and the energy of the nuclear electric quadrupole moment in the electric field gradient due to the distribution of charge within the atom. Molecular hyperfine structure is generally dominated by these two effects, but also includes the energy associated with the interaction between the magnetic moments associated with different magnetic nuclei in a molecule, as well as between the nuclear magnetic moments and the magnetic field generated by the rotation of the molecule. Hyperfine structure contrasts with '' fine structure'', which results from the interaction b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Absorption Cross-section
Absorption cross section is a measure for the probability of an absorption process. More generally, the term cross section is used in physics to quantify the probability of a certain particle-particle interaction, e.g., scattering, electromagnetic absorption, etc. (Note that light in this context is described as consisting of particles, i.e., photons.) In honor of the fundamental contribution of Maria Goeppert Mayer to this area, the unit for the two-photon absorption cross section is named the "GM". One GM is 10−50 cm4⋅s⋅photon−1. In the context of ozone shielding of ultraviolet light, absorption cross section is the ability of a molecule to absorb a photon of a particular wavelength and polarization. Analogously, in the context of nuclear engineering it refers to the probability of a particle (usually a neutron) being absorbed by a nucleus. Although the units are given as an area, it does not refer to an actual size area, at least partially because the density o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
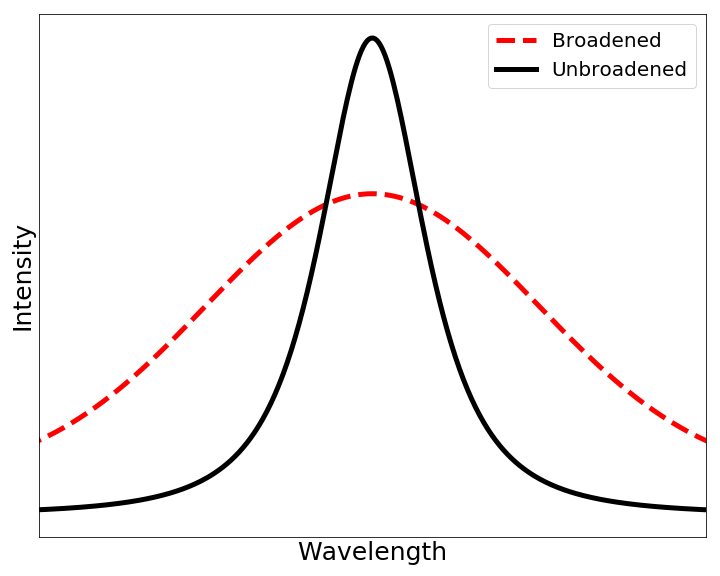
Doppler Broadening
In atomic physics, Doppler broadening is broadening of spectral lines due to the Doppler effect caused by a distribution of velocities of atoms or molecules. Different velocities of the emitting (or absorbing) particles result in different Doppler shifts, the cumulative effect of which is the emission (absorption) line broadening. This resulting line profile is known as a Doppler profile. A particular case is the thermal Doppler broadening due to the thermal motion of the particles. Then, the broadening depends only on the frequency of the spectral line, the mass of the emitting particles, and their temperature, and therefore can be used for inferring the temperature of an emitting (or absorbing) body being spectroscopically investigated. Derivation When a particle moves (e.g., due to the thermal motion) towards the observer, the emitted radiation is shifted to a higher frequency. Likewise, when the emitter moves away, the frequency is lowered. For non-relativistic thermal ve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spectral Hole Burning
Spectral hole burning is the frequency-selective bleaching of the absorption spectrum of a material, which leads to an increased transmission (a "spectral hole") at the selected frequency. Two basic requirements must be met for the phenomenon to be observed: # The spectrum is Inhomogeneous broadening, inhomogeneously broadened # The material undergoes, subsequent to light absorption, a modification which changes its absorption spectrum. Typical materials include dye molecules dissolved in suitable host matrices; the frequency-selective irradiation is usually realized by a narrow-band laser. Particular case Most molecules and atoms always return from the excited state to the initial ground state. In some situations, however, this may not happen. For example, some organic dye molecules can undergo a photochemical reaction, which alters the whole chemical structure of the molecule. If such a photochemically active molecule absorbs light, then with a probability of a few perce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Saturated Absorption Spectroscopy
In experimental atomic physics, saturated absorption spectroscopy or Doppler-free spectroscopy is a set-up that enables the precise determination of the transition frequency of an atom between its ground state and an optically excited state. The accuracy to which these frequencies can be determined is, ideally, limited only by the width of the excited state, which is the inverse of the lifetime of this state. However, the samples of atomic gas that are used for that purpose are generally at room temperature, where the measured frequency distribution is highly broadened due to the Doppler effect. Saturated absorption spectroscopy allows precise spectroscopy of the atomic levels without having to cool the sample down to temperatures at which the Doppler broadening is no longer relevant (which would be on the order of a few millikelvins). It is also used to lock the frequency of a laser to the precise wavelength of an atomic transition in atomic physics experiments. Doppler broadening o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spectroscopy
Spectroscopy is the field of study that measures and interprets the electromagnetic spectra that result from the interaction between electromagnetic radiation and matter as a function of the wavelength or frequency of the radiation. Matter waves and acoustic waves can also be considered forms of radiative energy, and recently gravitational waves have been associated with a spectral signature in the context of the Laser Interferometer Gravitational-Wave Observatory (LIGO) In simpler terms, spectroscopy is the precise study of color as generalized from visible light to all bands of the electromagnetic spectrum. Historically, spectroscopy originated as the study of the wavelength dependence of the absorption by gas phase matter of visible light dispersed by a prism. Spectroscopy, primarily in the electromagnetic spectrum, is a fundamental exploratory tool in the fields of astronomy, chemistry, materials science, and physics, allowing the composition, physical structure and e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |