|
Saline Drip
Intravenous therapy (abbreviated as IV therapy) is a medical technique that administers fluids, medications and nutrients directly into a person's vein. The intravenous route of administration is commonly used for rehydration or to provide nutrients for those who cannot, or will not—due to reduced mental states or otherwise—consume food or water by mouth. It may also be used to administer medications or other medical therapy such as blood products or electrolytes to correct electrolyte imbalances. Attempts at providing intravenous therapy have been recorded as early as the 1400s, but the practice did not become widespread until the 1900s after the development of techniques for safe, effective use. The intravenous route is the fastest way to deliver medications and fluid replacement throughout the body as they are introduced directly into the circulatory system and thus quickly distributed. For this reason, the intravenous route of administration is also used for the consumptio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cannula
A cannula (; Latin meaning 'little reed'; plural or ) is a tube that can be inserted into the body, often for the delivery or removal of fluid or for the gathering of samples. In simple terms, a cannula can surround the inner or outer surfaces of a trocar needle thus extending the effective needle length by at least half the length of the original needle. Its size mainly ranges from 14 to 24 gauge. Different-sized cannula have different colours as coded. Decannulation is the permanent removal of a cannula (extubation), especially of a tracheostomy cannula, once a physician determines it is no longer needed for breathing. Medicine Cannulas normally come with a trocar inside. The trocar is a needle, which punctures the body in order to get into the intended space. Many types of cannulas exist: Intravenous cannulas are the most common in hospital use. A variety of cannulas are used to establish cardiopulmonary bypass in cardiac surgery. A nasal cannula is a piece of plastic tu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Blood Substitutes
A blood substitute (also called artificial blood or blood surrogate) is a substance used to mimic and fulfill some functions of biological blood. It aims to provide an alternative to blood transfusion, which is transferring blood or blood-based products from one person into another. Thus far, there are no well-accepted ''oxygen-carrying'' blood substitutes, which is the typical objective of a red blood cell transfusion; however, there are widely available non-blood volume expanders for cases where only volume restoration is required. These are helping doctors and surgeons avoid the risks of disease transmission and immune suppression, address the chronic blood donor shortage, and address the concerns of Jehovah's Witnesses and others who have religious objections to receiving transfused blood. The main categories of "oxygen-carrying" blood substitutes being pursued are hemoglobin-based oxygen carriers (HBOC) and perfluorocarbon emulsions. Oxygen therapeutics are in clinical tri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Buffer Solution
A buffer solution (more precisely, pH buffer or hydrogen ion buffer) is an aqueous solution consisting of a mixture of a weak acid and its conjugate base, or vice versa. Its pH changes very little when a small amount of strong acid or base is added to it. Buffer solutions are used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications. In nature, there are many living systems that use buffering for pH regulation. For example, the bicarbonate buffering system is used to regulate the pH of blood, and bicarbonate also acts as a buffer in the ocean. Principles of buffering Buffer solutions resist pH change because of a chemical equilibrium between the weak acid HA and its conjugate base A−: When some strong acid is added to an equilibrium mixture of the weak acid and its conjugate base, hydrogen ions (H+) are added, and the equilibrium is shifted to the left, in accordance with Le Chatelier's principle. Because of this, the hydrogen io ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
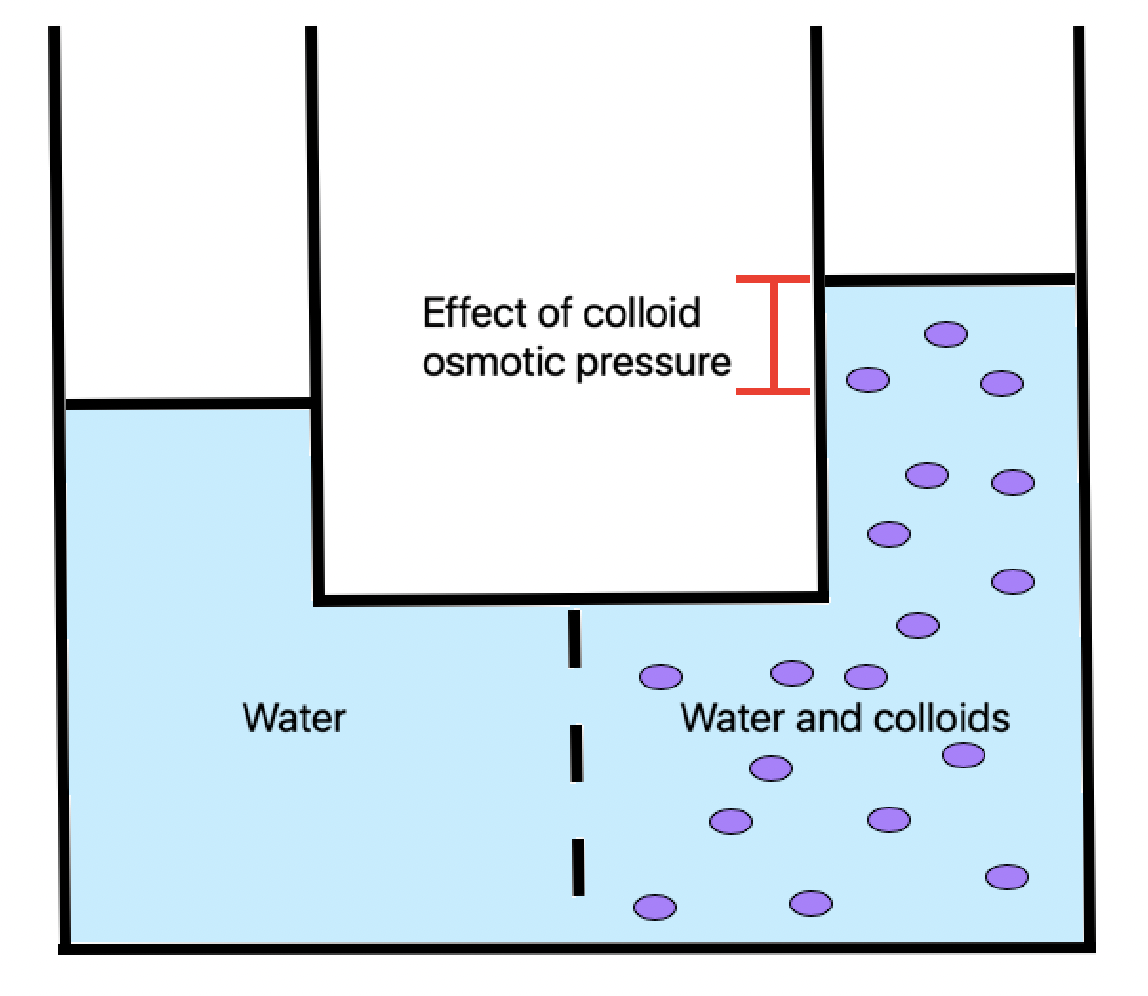
Colloid Osmotic Pressure
Oncotic pressure, or colloid osmotic-pressure, is a form of osmotic pressure induced by the proteins, notably albumin, in a blood vessel's plasma (blood/liquid) that causes a pull on fluid back into the capillary. Participating colloids displace water molecules, thus creating a relative water molecule deficit with water molecules moving back into the circulatory system within the lower venous pressure end of capillaries. It has the opposing effect of both hydrostatic blood pressure pushing water and small molecules out of the blood into the interstitial spaces within the arterial end of capillaries and interstitial colloidal osmotic pressure. These interacting factors determine the partition balancing of extracellular water between the blood plasma and outside the blood stream. Oncotic pressure strongly affects the physiological function of the circulatory system. It is suspected to have a major effect on the pressure across the glomerular filter. However, this concept has bee ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Burn
A burn is an injury to skin, or other tissues, caused by heat, cold, electricity, chemicals, friction, or ultraviolet radiation (like sunburn). Most burns are due to heat from hot liquids (called scalding), solids, or fire. Burns occur mainly in the home or the workplace. In the home, risks are associated with domestic kitchens, including stoves, flames, and hot liquids. In the workplace, risks are associated with fire and chemical and electric burns. Alcoholism and smoking are other risk factors. Burns can also occur as a result of self-harm or violence between people (assault). Burns that affect only the superficial skin layers are known as superficial or first-degree burns. They appear red without blisters and pain typically lasts around three days. When the injury extends into some of the underlying skin layer, it is a partial-thickness or second-degree burn. Blisters are frequently present and they are often very painful. Healing can require up to eight weeks and scarri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hypotonic
In chemical biology, tonicity is a measure of the effective osmotic pressure gradient; the water potential of two solutions separated by a partially-permeable cell membrane. Tonicity depends on the relative concentration of selective membrane-impermeable solutes across a cell membrane which determine the direction and extent of osmotic flux. It is commonly used when describing the swelling-versus-shrinking response of cells immersed in an external solution. Unlike osmotic pressure, tonicity is influenced only by solutes that cannot cross the membrane, as only these exert an effective osmotic pressure. Solutes able to freely cross the membrane do not affect tonicity because they will always equilibrate with equal concentrations on both sides of the membrane without net solvent movement. It is also a factor affecting imbibition. There are three classifications of tonicity that one solution can have relative to another: ''hypertonic'', ''hypotonic'', and ''isotonic''.A hypotonic so ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ringer's Lactate
Ringer's lactate solution (RL), also known as sodium lactate solution, Lactated Ringer’s, and Hartmann's solution, is a mixture of sodium chloride, sodium lactate, potassium chloride, and calcium chloride in water. It is used for replacing fluids and electrolytes in those who have low blood volume or low blood pressure. It may also be used to treat metabolic acidosis and to wash the eye following a chemical burn. It is given by intravenous infusion or applied to the affected area. Side effects may include allergic reactions, high blood potassium, hypervolemia, and high blood calcium. It may not be suitable for mixing with certain medications and some recommend against use in the same infusion as a blood transfusion. Ringer's lactate solution has a lower rate of acidosis as compared with normal saline. Use is generally safe in pregnancy and breastfeeding. Ringer's lactate solution is in the crystalloid family of medications. It is isotonic, i.e. it has the same tonicity as b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotonicity
In chemical biology, tonicity is a measure of the effective osmotic pressure gradient; the water potential of two solutions separated by a partially-permeable cell membrane. Tonicity depends on the relative concentration of selective membrane-impermeable solutes across a cell membrane which determine the direction and extent of osmotic flux. It is commonly used when describing the swelling-versus-shrinking response of cells immersed in an external solution. Unlike osmotic pressure, tonicity is influenced only by solutes that cannot cross the membrane, as only these exert an effective osmotic pressure. Solutes able to freely cross the membrane do not affect tonicity because they will always equilibrate with equal concentrations on both sides of the membrane without net solvent movement. It is also a factor affecting imbibition. There are three classifications of tonicity that one solution can have relative to another: ''hypertonic'', ''hypotonic'', and ''isotonic''.A hypotonic so ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Chloride
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g/mol respectively, 100 g of NaCl contains 39.34 g Na and 60.66 g Cl. Sodium chloride is the salt most responsible for the salinity of seawater and of the extracellular fluid of many multicellular organisms. In its edible form, salt (also known as ''table salt'') is commonly used as a condiment and food preservative. Large quantities of sodium chloride are used in many industrial processes, and it is a major source of sodium and chlorine compounds used as feedstocks for further chemical syntheses. Another major application of sodium chloride is de-icing of roadways in sub-freezing weather. Uses In addition to the familiar domestic uses of salt, more dominant applications of the approximately 250 million tonnes per year production (2008 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Saline (medicine)
Saline (also known as saline solution) is a mixture of sodium chloride (salt) and water. It has a number of uses in medicine including cleaning wounds, removal and storage of contact lenses, and help with dry eyes. By injection into a vein it is used to treat dehydration such as that from gastroenteritis and diabetic ketoacidosis. Large amounts may result in fluid overload, swelling, acidosis, and high blood sodium. In those with long-standing low blood sodium, excessive use may result in osmotic demyelination syndrome. Saline is in the crystalloid family of medications. It is most commonly used as a sterile 9 g of salt per litre (0.9%) solution, known as normal saline. Higher and lower concentrations may also occasionally be used. Saline is acidic, with a pH of 5.5 (due mainly to dissolved carbon dioxide). The medical use of saline began around 1831. It is on the World Health Organization's List of Essential Medicines. In 2020, sodium was the 274th most commonly p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Blood
Blood is a body fluid in the circulatory system of humans and other vertebrates that delivers necessary substances such as nutrients and oxygen to the cells, and transports metabolic waste products away from those same cells. Blood in the circulatory system is also known as ''peripheral blood'', and the blood cells it carries, ''peripheral blood cells''. Blood is composed of blood cells suspended in blood plasma. Plasma, which constitutes 55% of blood fluid, is mostly water (92% by volume), and contains proteins, glucose, mineral ions, hormones, carbon dioxide (plasma being the main medium for excretory product transportation), and blood cells themselves. Albumin is the main protein in plasma, and it functions to regulate the colloidal osmotic pressure of blood. The blood cells are mainly red blood cells (also called RBCs or erythrocytes), white blood cells (also called WBCs or leukocytes) and platelets (also called thrombocytes). The most abundant cells in vertebrate blo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gelatin
Gelatin or gelatine (from la, gelatus meaning "stiff" or "frozen") is a translucent, colorless, flavorless food ingredient, commonly derived from collagen taken from animal body parts. It is brittle when dry and rubbery when moist. It may also be referred to as hydrolyzed collagen, collagen hydrolysate, gelatine hydrolysate, hydrolyzed gelatine, and collagen peptides after it has undergone hydrolysis. It is commonly used as a gelling agent in food, beverages, medications, drug or vitamin capsules, photographic films, papers, and cosmetics. Substances containing gelatin or functioning in a similar way are called gelatinous substances. Gelatin is an irreversibly hydrolyzed form of collagen, wherein the hydrolysis reduces protein fibrils into smaller peptides; depending on the physical and chemical methods of denaturation, the molecular weight of the peptides falls within a broad range. Gelatin is present in gelatin desserts, most gummy candy and marshmallows, ice creams, dips ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |