|
Symmetry-adapted Perturbation Theory
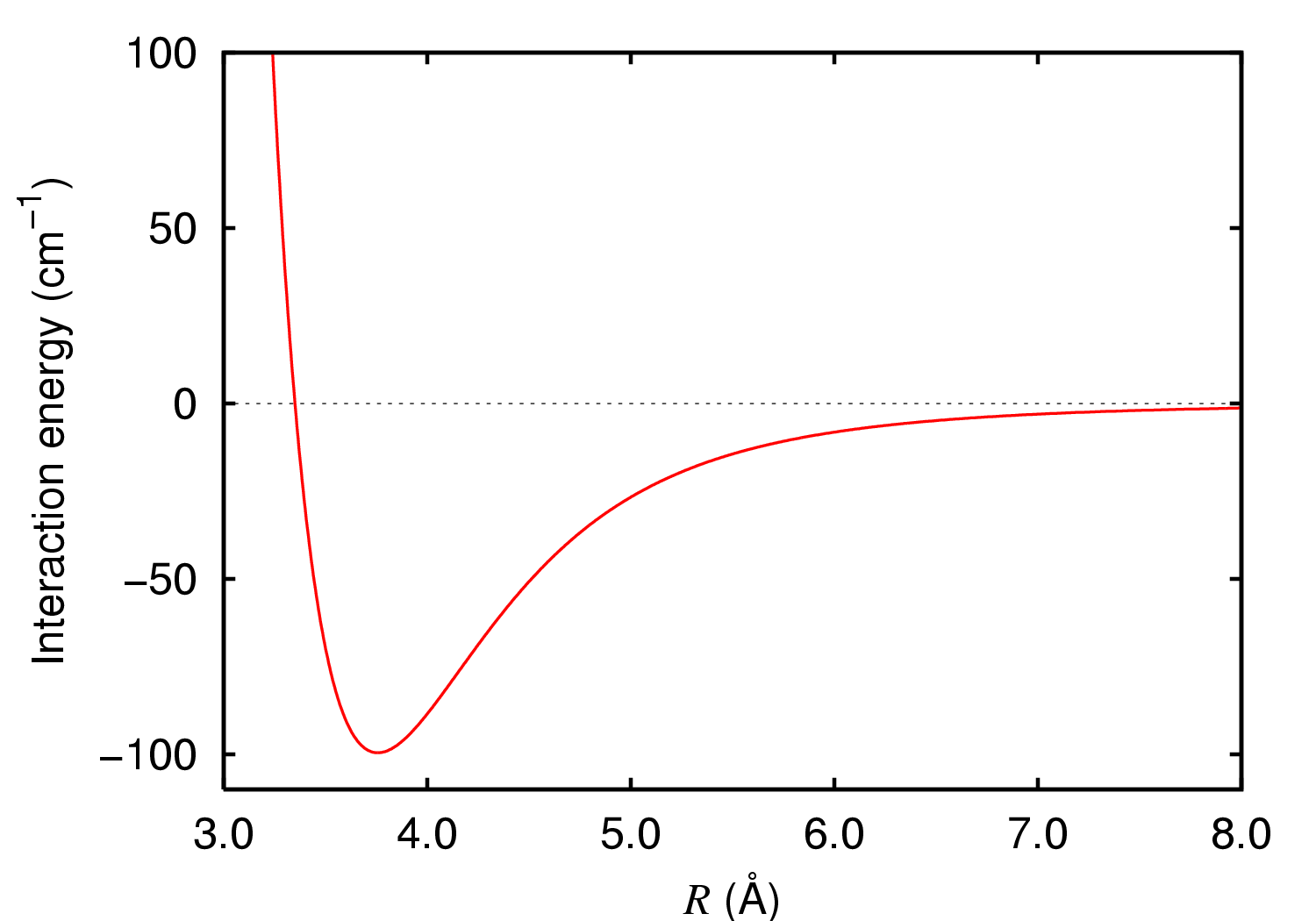
Symmetry-adapted perturbation theory or SAPT is a methodology in electronic structure theory developed to describe non-covalent interactions between atoms and/or molecules. SAPT is a member of the family of methods known as energy decomposition analysis (EDA). Most EDA methods decompose a total interaction energy that is computed via a ''supermolecular'' approach, such that: \Delta E_=E_-E_-E_ where \Delta E_ is the total interaction energy obtained via subtracting isolated monomer energies E_ and E_ from the dimer energy E_. A key deficiency of the supermolecular interaction energy is that it is susceptible to basis set superposition error (BSSE). The major difference between SAPT and supermolecular EDA methods is that, as the name suggests, SAPT computes the interaction energy ''directly'' via a perturbative approach. One consequence of capturing the total interaction energy as a perturbation to the total system energy rather than using the subtractive supermolecular method outli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electronic Structure
In quantum chemistry, electronic structure is the state of motion of electrons in an electrostatic field created by stationary nuclei. The term encompasses both the wave functions of the electrons and the energies associated with them. Electronic structure is obtained by solving quantum mechanical equations for the aforementioned clamped-nuclei problem. Electronic structure problems arise from the Born–Oppenheimer approximation. Along with nuclear dynamics, the electronic structure problem is one of the two steps in studying the quantum mechanical motion of a molecular system. Except for a small number of simple problems such as hydrogen-like atoms, the solution of electronic structure problems require modern computers. Electronic structure problem is routinely solved with quantum chemistry computer programs. Electronic structure calculations rank among the most computationally intensive tasks in all scientific calculations. For this reason, quantum chemistry calculatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Non-covalent Interactions
In chemistry, a non-covalent interaction differs from a covalent bond in that it does not involve the sharing of electrons, but rather involves more dispersed variations of electromagnetic interactions between molecules or within a molecule. The chemical energy released in the formation of non-covalent interactions is typically on the order of 1–5 kcal/ mol (1000–5000 calories per 6.02 molecules). Non-covalent interactions can be classified into different categories, such as electrostatic, π-effects, van der Waals forces, and hydrophobic effects. Non-covalent interactions are critical in maintaining the three-dimensional structure of large molecules, such as proteins and nucleic acids. In addition, they are also involved in many biological processes in which large molecules bind specifically but transiently to one another (see the properties section of the DNA page). These interactions also heavily influence drug design, crystallinity and design of materials, particularly f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atoms
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons. Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atoms are extremely small, typically around 100 picometers across. They are so small that accurately predicting their behavior using classical physics, as if they were tennis balls for example, is not possible due to quantum effects. More than 99.94% of an atom's mass is in the nucleus. The protons have a positive electric charge, the electrons have a negative electric charge, and the neutrons have no electric charge. If the number of protons and electrons are equal, then the atom is electrically neutral. If an atom has more or fewer electrons than protons, then it has an overall negative or positive charge, respectively – such atoms are called ions. The electrons of an atom are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecules
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and ''molecule'' is often used when referring to polyatomic ions. A molecule may be homonuclear, that is, it consists of atoms of one chemical element, e.g. two atoms in the oxygen molecule (O2); or it may be heteronuclear, a chemical compound composed of more than one element, e.g. water (molecule), water (two hydrogen atoms and one oxygen atom; H2O). In the kinetic theory of gases, the term ''molecule'' is often used for any gaseous particle regardless of its composition. This relaxes the requirement that a molecule contains two or more atoms, since the noble gases are individual atoms. Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic bonds, are typic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Basis Set Superposition Error
In quantum chemistry, calculations using finite basis sets are susceptible to basis set superposition error (BSSE). As the atoms of interacting molecules (or of different parts of the same molecule - intramolecular BSSE) approach one another, their basis functions overlap. Each monomer "borrows" functions from other nearby components, effectively increasing its basis set and improving the calculation of derived properties such as energy. If the total energy is minimised as a function of the system geometry, the short-range energies from the mixed basis sets must be compared with the long-range energies from the unmixed sets, and this mismatch introduces an error. Other than using infinite basis sets, two methods exist to eliminate the BSSE. In the ''chemical Hamiltonian approach'' (CHA), basis set mixing is prevented ''a priori'', by replacing the conventional Hamiltonian with one in which all the projector-containing terms that would allow mixing have been removed. In the ''cou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perturbative
In quantum mechanics, perturbation theory is a set of approximation schemes directly related to mathematical perturbation for describing a complicated quantum system in terms of a simpler one. The idea is to start with a simple system for which a mathematical solution is known, and add an additional "perturbing" Hamiltonian representing a weak disturbance to the system. If the disturbance is not too large, the various physical quantities associated with the perturbed system (e.g. its energy levels and eigenstates) can be expressed as "corrections" to those of the simple system. These corrections, being small compared to the size of the quantities themselves, can be calculated using approximate methods such as asymptotic series. The complicated system can therefore be studied based on knowledge of the simpler one. In effect, it is describing a complicated unsolved system using a simple, solvable system. Approximate Hamiltonians Perturbation theory is an important tool for d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrostatic Interaction
Electrostatics is a branch of physics that studies electric charges at rest (static electricity). Since classical times, it has been known that some materials, such as amber, attract lightweight particles after rubbing. The Greek word for amber, (), was thus the source of the word 'electricity'. Electrostatic phenomena arise from the forces that electric charges exert on each other. Such forces are described by Coulomb's law. Even though electrostatically induced forces seem to be rather weak, some electrostatic forces are relatively large. The force between an electron and a proton, which together make up a hydrogen atom, is about 36 orders of magnitude stronger than the gravitational force acting between them. There are many examples of electrostatic phenomena, from those as simple as the attraction of plastic wrap to one's hand after it is removed from a package, to the apparently spontaneous explosion of grain silos, the damage of electronic components during manufactur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pauli Repulsion
In chemistry and physics, the exchange interaction (with an exchange energy and exchange term) is a quantum mechanical effect that only occurs between identical particles. Despite sometimes being called an exchange force in an analogy to classical force, it is not a true force as it lacks a force carrier. The effect is due to the wave function of indistinguishable particles being subject to exchange symmetry, that is, either remaining unchanged (symmetric) or changing sign (antisymmetric) when two particles are exchanged. Both bosons and fermions can experience the exchange interaction. For fermions, this interaction is sometimes called Pauli repulsion and is related to the Pauli exclusion principle. For bosons, the exchange interaction takes the form of an effective attraction that causes identical particles to be found closer together, as in Bose–Einstein condensation. The exchange interaction alters the expectation value of the distance when the wave functions of two or more ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrostatic Induction
Electrostatic induction, also known as "electrostatic influence" or simply "influence" in Europe and Latin America, is a redistribution of electric charge in an object that is caused by the influence of nearby charges. In the presence of a charged body, an insulated conductor develops a positive charge on one end and a negative charge on the other end. Induction was discovered by British scientist John Canton in 1753 and Swedish professor Johan Carl Wilcke in 1762. Electrostatic generators, such as the Wimshurst machine, the Van de Graaff generator and the electrophorus, use this principle. See also Stephen Gray in this context. Due to induction, the electrostatic potential (voltage) is constant at any point throughout a conductor. Electrostatic induction is also responsible for the attraction of light nonconductive objects, such as balloons, paper or styrofoam scraps, to static electric charges. Electrostatic induction laws apply in dynamic situations as far as the quasistat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Orbitals
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The terms ''atomic orbital'' and ''molecular orbital'' were introduced by Robert S. Mulliken in 1932 to mean ''one-electron orbital wave functions''. At an elementary level, they are used to describe the ''region'' of space in which a function has a significant amplitude. In an isolated atom, the orbital electrons' location is determined by functions called atomic orbitals. When multiple atoms combine chemically into a molecule, the electrons' locations are determined by the molecule as a whole, so the atomic orbitals combine to form molecular orbitals. The electrons from the constituent atoms occupy the molecular orbitals. Mathematically, molecular orbitals are an approximate solution to the Schrödin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
London Dispersion
London dispersion forces (LDF, also known as dispersion forces, London forces, instantaneous dipole–induced dipole forces, fluctuating induced dipole bonds or loosely as van der Waals forces) are a type of intermolecular force acting between atoms and molecules that are normally electrically symmetric; that is, the electrons are symmetrically distributed with respect to the nucleus. They are part of the van der Waals forces. The LDF is named after the German physicist Fritz London. They are the weakest intermolecular force. Introduction The electron distribution around an atom or molecule undergoes fluctuations in time. These fluctuations create instantaneous electric fields which are felt by other nearby atoms and molecules, which in turn adjust the spatial distribution of their own electrons. The net effect is that the fluctuations in electron positions in one atom induce a corresponding redistribution of electrons in other atoms, such that the electron motions become corre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |