|
Sulphide Ore
Sulfide (British English also sulphide) is an inorganic anion of sulfur with the chemical formula S2ŌłÆ or a compound containing one or more S2ŌłÆ ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to chemical compounds large families of inorganic and organic compounds, e.g. lead sulfide and dimethyl sulfide. Hydrogen sulfide (H2S) and bisulfide (SHŌłÆ) are the conjugate acids of sulfide. Chemical properties The sulfide ion, S2ŌłÆ, does not exist in aqueous alkaline solutions of Na2S. Instead sulfide converts to hydrosulfide: :S2ŌłÆ + H2O ŌåÆ SHŌłÆ + OHŌłÆ Upon treatment with an acid, sulfide salts convert to hydrogen sulfide: :S2ŌłÆ + H+ ŌåÆ SHŌłÆ :SHŌłÆ + H+ ŌåÆ H2S Oxidation of sulfide is a complicated process. Depending on the conditions, the oxidation can produce elemental sulfur, polysulfides, polythionates, sulfite, or sulfate. Metal sulfides react with halogens, forming sulfur and metal salts. :8 MgS + 8 I2 ŌåÆ S8 + 8&nbs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bisulfide
Bisulfide (or bisulphide in British English) is an inorganic anion with the chemical formula HSŌłÆ (also written as SHŌłÆ). It contributes no color to bisulfide salts, and its salts may have a distinctive putrid smell. It is a strong base. Bisulfide solutions are corrosive and attack the skin. It is an important chemical reagent and an industrial chemical, mainly used in paper pulp industry (Kraft process), textiles, synthetic flavors, coloring brasses, and iron control. Properties A variety of salts are known, including sodium hydrosulfide and potassium hydrosulfide. Ammonium hydrosulfide, a component of "stink bombs" has not been isolated as a pure solid. Some compounds described as salts of the sulfide dianion contain primarily hydrosulfide. For example, the hydrated form of sodium sulfide, nominally with the formula , is better described as . Aqueous bisulfide absorbs light at around 230 nm in the UVŌĆōvisible spectrum. Using this approach, bisulfide has been de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Halogen
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group is known as group 17. The word "halogen" means "salt former" (or "salt maker"). When halogens react with metals, they produce a wide range of salts, including calcium fluoride, sodium chloride (common table salt), silver bromide and potassium iodide. The group of halogens is the only periodic table group that contains elements in three of the main states of matter at standard temperature and pressure. All of the halogens form acids when bonded to hydrogen. Most halogens are typically produced from minerals or salts. The middle halogensŌĆöchlorine, bromine, and iodineŌĆöare often used as disinfectants. Organobromides are the most important class of flame retardants, while elemental halogens are dangerous and can be toxic. History The fl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lead
Lead is a chemical element with the symbol Pb (from the Latin ) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cut, lead is a shiny gray with a hint of blue. It tarnishes to a dull gray color when exposed to air. Lead has the highest atomic number of any stable element and three of its isotopes are endpoints of major nuclear decay chains of heavier elements. Lead is toxic, even in small amounts, especially to children. Lead is a relatively unreactive post-transition metal. Its weak metallic character is illustrated by its amphoteric nature; lead and lead oxides react with acids and bases, and it tends to form covalent bonds. Compounds of lead are usually found in the +2 oxidation state rather than the +4 state common with lighter members of the carbon group. Exceptions are mostly limited to organolead compounds. Like the lighter members of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Galena
Galena, also called lead glance, is the natural mineral form of lead(II) sulfide (PbS). It is the most important ore of lead and an important source of silver. Galena is one of the most abundant and widely distributed sulfide minerals. It crystallizes in the cubic crystal system often showing octahedral forms. It is often associated with the minerals sphalerite, calcite and fluorite. Occurrence Galena is the main ore of lead, used since ancient times, since lead can be smelted from galena in an ordinary wood fire. Galena typically is found in hydrothermal veins in association with sphalerite, marcasite, chalcopyrite, cerussite, anglesite, dolomite, calcite, quartz, barite, and fluorite. It is also found in association with sphalerite in low-temperature lead-zinc deposits within limestone beds. Minor amounts are found in contact metamorphic zones, in pegmatites, and disseminated in sedimentary rock. In some deposits the galena contains up to 0.5% silver, a byproduct that ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mercury (element)
Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver and was formerly named hydrargyrum ( ) from the Greek words, ''hydor'' (water) and ''argyros'' (silver). A heavy, silvery d-block A block of the periodic table is a set of elements unified by the atomic orbitals their valence electrons or vacancies lie in. The term appears to have been first used by Charles Janet. Each block is named after its characteristic orbital: s-blo ... element, mercury is the only metallic element that is known to be liquid at standard temperature and pressure; the only other element that is liquid under these conditions is the halogen bromine, though metals such as caesium, gallium, and rubidium melt just above room temperature. Mercury occurs in deposits throughout the world mostly as cinnabar (mercuric sulfide). The red pigment vermilion is obtained by Mill (grinding), grinding natural cinnabar or synthetic mercuric sulfide. Mercury is used in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cinnabar
Cinnabar (), or cinnabarite (), from the grc, ╬║╬╣╬Į╬Į╬¼╬▓╬▒Žü╬╣ (), is the bright scarlet to brick-red form of Mercury sulfide, mercury(II) sulfide (HgS). It is the most common source ore for refining mercury (element), elemental mercury and is the historic source for the brilliant red or scarlet pigment termed vermilion and associated red mercury pigments. Cinnabar generally occurs as a vein-filling mineral associated with recent volcanic activity and alkaline hot springs. The mineral resembles quartz in symmetry and in its exhibiting birefringence. Cinnabar has a mean refractive index near 3.2, a mohs scale of mineral hardness, hardness between 2.0 and 2.5, and a specific gravity of approximately 8.1. The color and properties derive from a structure that is a hexagonal crystalline bravais lattice, lattice belonging to the trigonal crystal system, crystals that sometimes exhibit Crystal twinning, twinning. Cinnabar has been used for its color since antiquity in the Near East ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silver
Silver is a chemical element with the Symbol (chemistry), symbol Ag (from the Latin ', derived from the Proto-Indo-European wikt:Reconstruction:Proto-Indo-European/hŌééerŪĄ-, ''hŌééerŪĄ'': "shiny" or "white") and atomic number 47. A soft, white, lustrous transition metal, it exhibits the highest electrical conductivity, thermal conductivity, and reflectivity of any metal. The metal is found in the Earth's crust in the pure, free elemental form ("native silver"), as an alloy with gold and other metals, and in minerals such as argentite and chlorargyrite. Most silver is produced as a byproduct of copper, gold, lead, and zinc Refining (metallurgy), refining. Silver has long been valued as a precious metal. Silver metal is used in many bullion coins, sometimes bimetallism, alongside gold: while it is more abundant than gold, it is much less abundant as a native metal. Its purity is typically measured on a per-mille basis; a 94%-pure alloy is described as "0.940 fine". As one of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Argentite
In mineralogy, argentite (from the Latin ''argentum'', silver) is cubic silver sulfide (Ag2S), which can only exist at temperatures above 173 ┬░C, 177 ┬░C or 179 ┬░C. When it cools to ordinary temperatures it turns into its monoclinic polymorph, acanthite. The International Mineralogical Association has decided to reject argentite as a proper mineral. The name "argentite" sometimes also refers to pseudomorphs of argentite: specimens of acanthite which still display some of the outward signs of the cubic crystal form, even though their actual crystal structure is monoclinic due to the lower temperature. This form of acanthite is occasionally found as uneven cubes and octahedra, but more often as dendritic or earthy masses, with a blackish lead-grey color and metallic luster. Argentite belongs to the galena group. Cleavage, which is so prominent a feature in galena, here presents only in traces. The mineral is perfectly sectile and has a shining streak; hardness 2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metal
A metal (from Greek ╬╝╬ŁŽä╬▒╬╗╬╗╬┐╬Į ''m├®tallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typically ductile (can be drawn into wires) and malleable (they can be hammered into thin sheets). These properties are the result of the ''metallic bond'' between the atoms or molecules of the metal. A metal may be a chemical element such as iron; an alloy such as stainless steel; or a molecular compound such as polymeric sulfur nitride. In physics, a metal is generally regarded as any substance capable of conducting electricity at a temperature of absolute zero. Many elements and compounds that are not normally classified as metals become metallic under high pressures. For example, the nonmetal iodine gradually becomes a metal at a pressure of between 40 and 170 thousand times atmospheric pressure. Equally, some materials regarded as metals ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
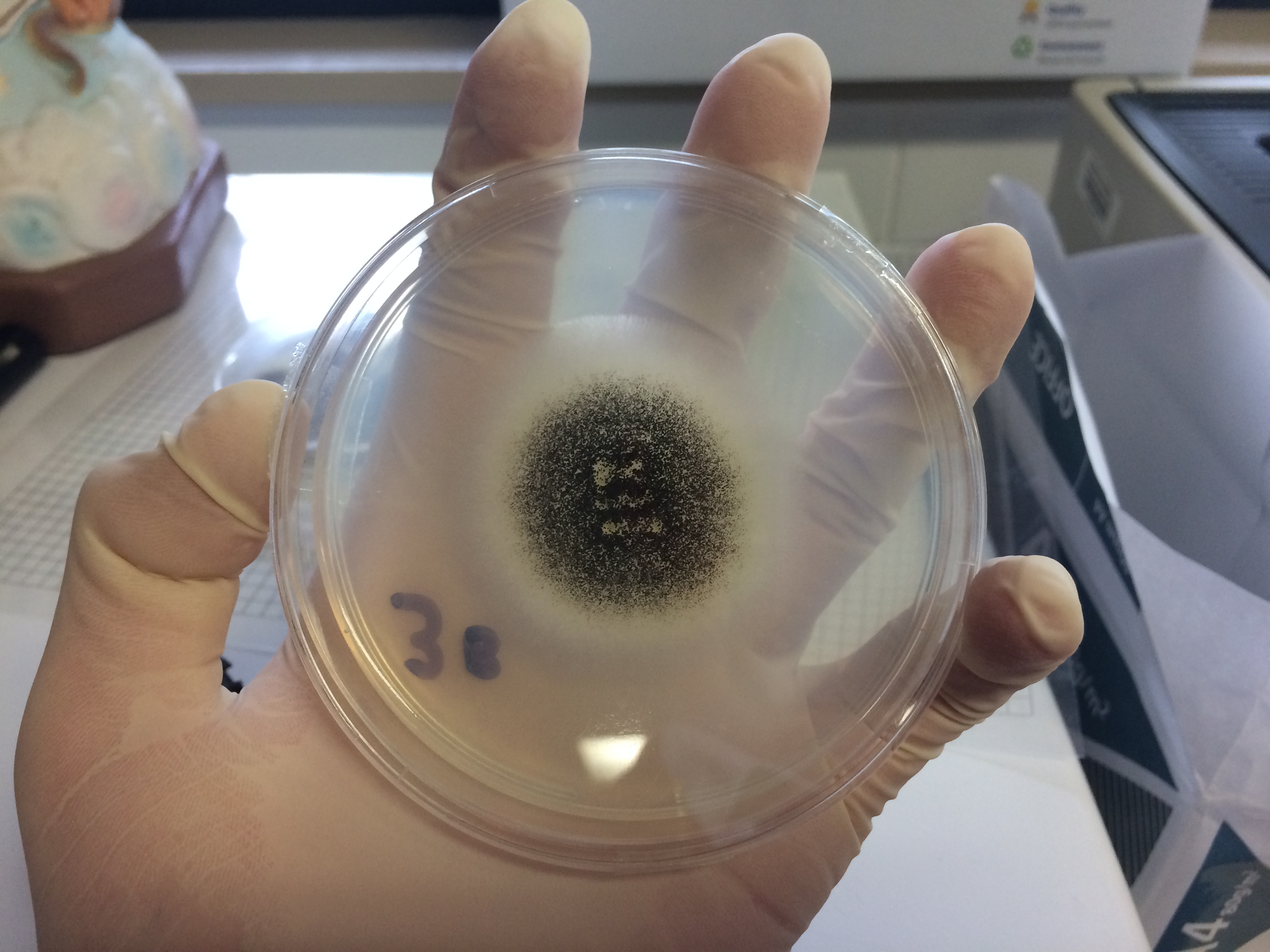
Aspergillus Niger
''Aspergillus niger'' is a mold classified within the ''Nigri'' section of the ''Aspergillus'' genus. The ''Aspergillus'' genus consists of common molds found throughout the environment within soil and water, on vegetation, in fecal matter, on decomposing matter, and suspended in the air. Species within this genus often grow quickly and can sporulate within a few days of germination. A combination of characteristics unique to ''A. niger'' makes the microbe invaluable to the production of many acids, proteins and bioactive compounds. Characteristics including extensive metabolic diversity, high production yield, secretion capability, and the ability to conduct post-translational modifications are responsible for ''A. niger's'' robust production of secondary metabolites. ''A. niger's'' capability to withstand extremely acidic conditions makes it especially important to the industrial production of citric acid. ''A. niger'' causes a disease known as "black mold" on certain fruits an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Semiconductor
A semiconductor is a material which has an electrical resistivity and conductivity, electrical conductivity value falling between that of a electrical conductor, conductor, such as copper, and an insulator (electricity), insulator, such as glass. Its electrical resistivity and conductivity, resistivity falls as its temperature rises; metals behave in the opposite way. Its conducting properties may be altered in useful ways by introducing impurities ("doping (semiconductor), doping") into the crystal structure. When two differently doped regions exist in the same crystal, a semiconductor junction is created. The behavior of charge carriers, which include electrons, ions, and electron holes, at these junctions is the basis of diodes, transistors, and most modern electronics. Some examples of semiconductors are silicon, germanium, gallium arsenide, and elements near the so-called "metalloid staircase" on the periodic table. After silicon, gallium arsenide is the second-most common s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cadmium Yellow
Cadmium pigments are a class of pigments that contain cadmium. Most of the cadmium produced worldwide has been for use in rechargeable nickelŌĆōcadmium batteries, which have been replaced by other rechargeable nickel-chemistry cell varieties such as NiMH cells, but about half of the remaining consumption of cadmium, which is approximately annually, is used to produce colored cadmium pigments. The principal pigments are a family of yellow, orange and red cadmium sulfides and sulfoselenides, as well as compounds with other metals. Cadmium is toxic to humans and other animals in very small amounts, especially when it is inhaled, which often happens when working with powdered pigment or breathing the dust from chalk pastels. As a result, it is not appropriate for children to use any art supplies that contain cadmium pigments. However, because the pigments have some desirable qualities, such as resistance to fading, some adult artists continue to use them. Artists' paints ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |