|
Sulfur Isotope
Sulfur (16S) has 23 known isotopes with mass numbers ranging from 27 to 49, four of which are stable: 32S (95.02%), 33S (0.75%), 34S (4.21%), and 36S (0.02%). The preponderance of sulfur-32 is explained by its production from carbon-12 plus successive fusion capture of five helium-4 nuclei, in the so-called alpha process of exploding type II supernovas (see silicon burning). Other than 35S, the radioactive isotopes of sulfur are all comparatively short-lived. 35S is formed from cosmic ray spallation of 40 Ar in the atmosphere. It has a half-life of 87 days. The next longest-lived radioisotope is sulfur-38, with a half-life of 170 minutes. The shortest-lived is 49S, with a half-life shorter than 200 nanoseconds. Heavier radioactive isotopes of sulfur decay to chlorine. When sulfide minerals are precipitated, isotopic equilibration among solids and liquid may cause small differences in the δ34S values of co-genetic minerals. The differences between minerals can be used to estima ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur Isotope Biogeochemistry
Sulfur isotope biogeochemistry is the study of the distribution of sulfur isotopes in biological and geological materials. In addition to its common isotope, 32S, sulfur has three rare stable isotopes: 34S, 36S, and 33S. The distribution of these isotopes in the environment is controlled by many biochemical and physical processes, including biological metabolisms, mineral formation processes, and atmospheric chemistry. Measuring the abundance of sulfur stable isotopes in natural materials, like bacterial cultures, minerals, or seawater, can reveal information about these processes both in the modern environment and over Earth history. Background Natural abundance of sulfur isotopes Sulfur has 24 known isotopes, 4 of which are stable (meaning that they do not undergo radioactive decay). 32S, the common isotope of sulfur, makes up 95.0% of the natural sulfur on Earth. In the atomic symbol of 32S, the number 32 refers to the mass of each sulfur atom in Daltons, the result of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula S8. Elemental sulfur is a bright yellow, crystalline solid at room temperature. Sulfur is the tenth most abundant element by mass in the universe and the fifth most on Earth. Though sometimes found in pure, native form, sulfur on Earth usually occurs as sulfide and sulfate minerals. Being abundant in native form, sulfur was known in ancient times, being mentioned for its uses in ancient India, ancient Greece, China, and ancient Egypt. Historically and in literature sulfur is also called brimstone, which means "burning stone". Today, almost all elemental sulfur is produced as a byproduct of removing sulfur-containing contaminants from natural gas and petroleum.. Downloahere The greatest commercial use of the element is the production ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
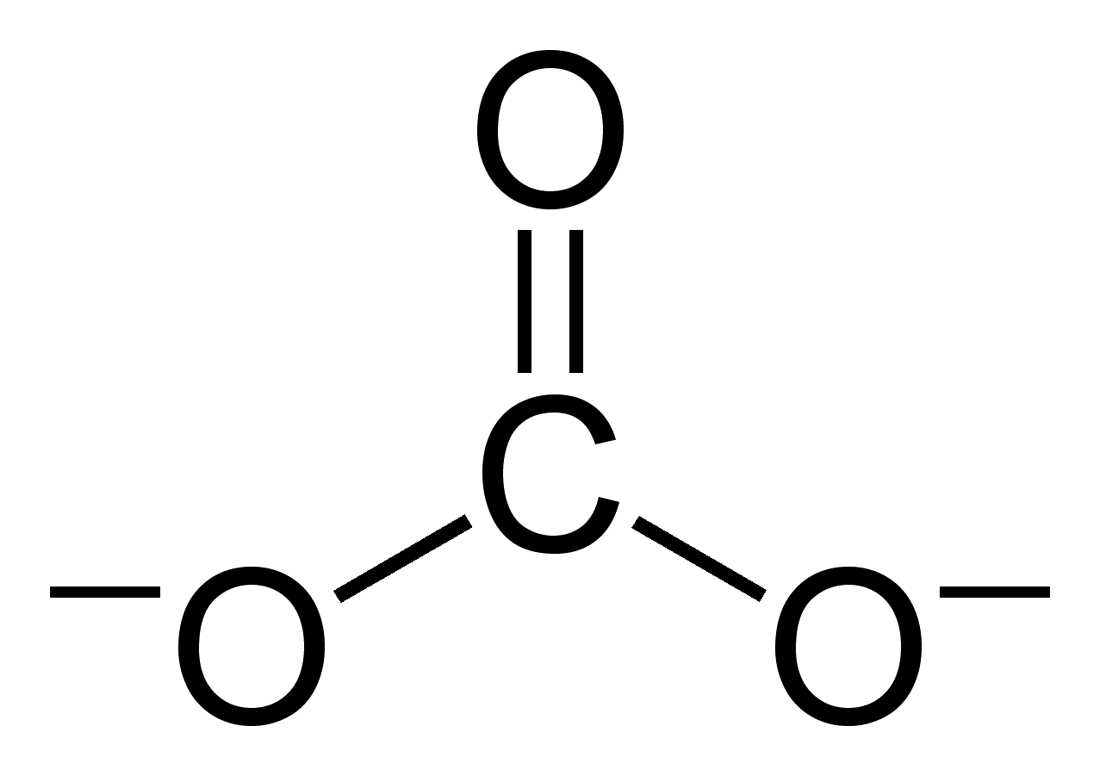
Carbonate
A carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word ''carbonate'' may also refer to a carbonate ester, an organic compound containing the carbonate group C(=O)(O–)2. The term is also used as a verb, to describe carbonation: the process of raising the concentrations of carbonate and bicarbonate ions in water to produce carbonated water and other carbonated beverageseither by the addition of carbon dioxide gas under pressure or by dissolving carbonate or bicarbonate salts into the water. In geology and mineralogy, the term "carbonate" can refer both to carbonate minerals and carbonate rock (which is made of chiefly carbonate minerals), and both are dominated by the carbonate ion, . Carbonate minerals are extremely varied and ubiquitous in chemically precipitated sedimentary rock. The most common are calcite or calcium carbonate, CaCO3, the chief constituent of limestone (as well ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cosmogenic Nuclide
Cosmogenic nuclides (or cosmogenic isotopes) are rare nuclides (isotopes) created when a high-energy cosmic ray interacts with the nucleus of an '' in situ'' Solar System atom, causing nucleons (protons and neutrons) to be expelled from the atom (see cosmic ray spallation). These nuclides are produced within Earth materials such as rocks or soil, in Earth's atmosphere, and in extraterrestrial items such as meteoroids. By measuring cosmogenic nuclides, scientists are able to gain insight into a range of geological and astronomical processes. There are both radioactive and stable cosmogenic nuclides. Some of these radionuclides are tritium, carbon-14 and phosphorus-32. Certain light (low atomic number) primordial nuclides (isotopes of lithium, beryllium and boron) are thought to have been created not only during the Big Bang, but also (and perhaps primarily) to have been made after the Big Bang, but before the condensation of the Solar System, by the process of cosmic ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta Decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide. For example, beta decay of a neutron transforms it into a proton by the emission of an electron accompanied by an antineutrino; or, conversely a proton is converted into a neutron by the emission of a positron with a neutrino in so-called ''positron emission''. Neither the beta particle nor its associated (anti-)neutrino exist within the nucleus prior to beta decay, but are created in the decay process. By this process, unstable atoms obtain a more stable ratio of protons to neutrons. The probability of a nuclide decaying due to beta and other forms of decay is determined by its nuclear binding energy. The binding energies of all existing nuclides form what is called the nuclear band or valley of stability. For either electron or positron e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Halo Nucleus
In nuclear physics, an atomic nucleus is called a halo nucleus or is said to have a nuclear halo when it has a core nucleus surrounded by a "halo" of orbiting protons or neutrons, which makes the radius of the nucleus appreciably larger than that predicted by the liquid drop model. Halo nuclei form at the extreme edges of the table of nuclides — the neutron drip line and proton drip line — and have short half-lives, measured in milliseconds. These nuclei are studied shortly after their formation in an ion beam. Typically, an atomic nucleus is a tightly bound group of protons and neutrons. However, in some nuclides, there is an overabundance of one species of nucleon. In some of these cases, a nuclear core and a halo will form. Often, this property may be detected in scattering experiments, which show the nucleus to be much larger than the otherwise expected value. Normally, the cross-section (corresponding to the classical radius) of the nucleus is proportional to the cube ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rocky Mountain
The Rocky Mountains, also known as the Rockies, are a major mountain range and the largest mountain system in North America. The Rocky Mountains stretch in straight-line distance from the northernmost part of western Canada, to New Mexico in the southwestern United States. Depending on differing definitions between Canada and the U.S., its northern terminus is located either in northern British Columbia's Terminal Range south of the Liard River and east of the Trench, or in the northeastern foothills of the Brooks Range/ British Mountains that face the Beaufort Sea coasts between the Canning River and the Firth River across the Alaska- Yukon border. Its southernmost point is near the Albuquerque area adjacent to the Rio Grande rift and north of the Sandia–Manzano Mountain Range. Being the easternmost portion of the North American Cordillera, the Rockies are distinct from the tectonically younger Cascade Range and Sierra Nevada, which both lie farther to its west. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Natural Abundance
In physics, natural abundance (NA) refers to the abundance of isotopes of a chemical element as naturally found on a planet. The relative atomic mass (a weighted average, weighted by mole-fraction abundance figures) of these isotopes is the atomic weight listed for the element in the periodic table. The abundance of an isotope varies from planet to planet, and even from place to place on the Earth, but remains relatively constant in time (on a short-term scale). As an example, uranium has three naturally occurring isotopes: 238U, 235U and 234U. Their respective natural mole-fraction abundances are 99.2739–99.2752%, 0.7198–0.7202%, and 0.0050–0.0059%. For example, if 100,000 uranium atoms were analyzed, one would expect to find approximately 99,274 238U atoms, approximately 720 235U atoms, and very few (most likely 5 or 6) 234U atoms. This is because 238U is much more stable than 235U or 234U, as the half-life of each isotope reveals: 4.468 × 109 years for 238U c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrology
Hydrology () is the scientific study of the movement, distribution, and management of water on Earth and other planets, including the water cycle, water resources, and environmental watershed sustainability. A practitioner of hydrology is called a hydrologist. Hydrologists are scientists studying earth or environmental science, civil or environmental engineering, and physical geography. Using various analytical methods and scientific techniques, they collect and analyze data to help solve water related problems such as environmental preservation, natural disasters, and water management. Hydrology subdivides into surface water hydrology, groundwater hydrology (hydrogeology), and marine hydrology. Domains of hydrology include hydrometeorology, surface hydrology, hydrogeology, drainage-basin management, and water quality, where water plays the central role. Oceanography and meteorology are not included because water is only one of many important aspects within those field ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Forest
A forest is an area of land dominated by trees. Hundreds of definitions of forest are used throughout the world, incorporating factors such as tree density, tree height, land use, legal standing, and ecological function. The United Nations' Food and Agriculture Organization (FAO) defines a forest as, "Land spanning more than 0.5 hectares with trees higher than 5 meters and a canopy cover of more than 10 percent, or trees able to reach these thresholds ''in situ''. It does not include land that is predominantly under agricultural or urban use." Using this definition, '' Global Forest Resources Assessment 2020'' (FRA 2020) found that forests covered , or approximately 31 percent of the world's land area in 2020. Forests are the predominant terrestrial ecosystem of Earth, and are found around the globe. More than half of the world's forests are found in only five countries (Brazil, Canada, China, Russia, and the United States). The largest share of forests (45 percent) are in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fugacity
In chemical thermodynamics, the fugacity of a real gas is an effective partial pressure which replaces the mechanical partial pressure in an accurate computation of the chemical equilibrium constant. It is equal to the pressure of an ideal gas which has the same temperature and molar Gibbs free energy as the real gas. Fugacities are determined experimentally or estimated from various models such as a Van der Waals gas that are closer to reality than an ideal gas. The real gas pressure and fugacity are related through the dimensionless fugacity coefficient . \varphi = \frac For an ideal gas, fugacity and pressure are equal and so . Taken at the same temperature and pressure, the difference between the molar Gibbs free energies of a real gas and the corresponding ideal gas is equal to . The fugacity is closely related to the thermodynamic activity. For a gas, the activity is simply the fugacity divided by a reference pressure to give a dimensionless quantity. This reference pres ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. Oxygen is Earth's most abundant element, and after hydrogen and helium, it is the third-most abundant element in the universe. At standard temperature and pressure, two atoms of the element bind to form dioxygen, a colorless and odorless diatomic gas with the formula . Diatomic oxygen gas currently constitutes 20.95% of the Earth's atmosphere, though this has changed considerably over long periods of time. Oxygen makes up almost half of the Earth's crust in the form of oxides.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. Many major classes of organic molecules in living organisms contain oxygen atoms, such as proteins, nucleic acids, carbohydrates, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |