|
Subtilase
Subtilases are a protein family, family of subtilisin-like serine proteases. They appear to have independently and convergently evolved an Aspartate, Asp/Serine, Ser/Histidine, His catalytic triad, like in the trypsin, trypsin serine proteases. The structure of proteins in this family shows that they have an alpha/beta fold containing a 7-stranded parallel beta sheet. The subtilisin family is the second largest serine protease family characterised to date. Over 200 subtilases are presently known, more than 170 of which with their complete amino acid sequence. Subtilase is widespread, being found in eubacteria, archaebacteria, eukaryotes and viruses. The vast majority of the family are endopeptidases, although there is an exopeptidase, tripeptidyl peptidase. Structures have been determined for several members of the subtilisin family showing that subtilisins exploit the same catalytic triad as the chymotrypsins although the residues occur in a different order (His/Asp/Ser in chymotr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Family
A protein family is a group of evolutionarily related proteins. In many cases, a protein family has a corresponding gene family, in which each gene encodes a corresponding protein with a 1:1 relationship. The term "protein family" should not be confused with Family (biology), family as it is used in taxonomy. Proteins in a family descend from a common ancestor and typically have similar protein structure, three-dimensional structures, functions, and significant Sequence homology, sequence similarity. The most important of these is sequence similarity (usually amino-acid sequence), since it is the strictest indicator of homology and therefore the clearest indicator of common ancestry. A fairly well developed framework exists for evaluating the significance of similarity between a group of sequences using sequence alignment methods. Proteins that do not share a common ancestor are very unlikely to show statistically significant sequence similarity, making sequence alignment a powerf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Furin
Furin is a protease, a proteolytic enzyme that in humans and other animals is encoded by the ''FURIN'' gene. Some proteins are inactive when they are first synthesized, and must have sections removed in order to become active. Furin cleaves these sections and activates the proteins. It was named furin because it was in the upstream region of an oncogene known as FES. The gene was known as FUR (FES Upstream Region) and therefore the protein was named furin. Furin is also known as PACE (Paired basic Amino acid Cleaving Enzyme). A member of family S8, furin is a subtilisin-like peptidase. Function The protein encoded by this gene is an enzyme that belongs to the subtilisin-like proprotein convertase family. The members of this family are proprotein convertases that process latent precursor proteins into their biologically active products. This encoded protein is a calcium-dependent serine endoprotease that can efficiently cleave precursor proteins at their paired basic amino acid pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
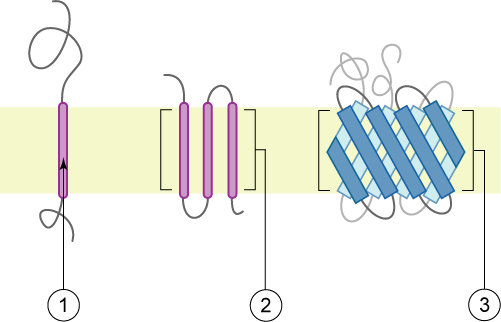
Single-pass Transmembrane Proteins
A single-pass membrane protein also known as single-spanning protein or bitopic protein is a transmembrane protein that spans the lipid bilayer only once. These proteins may constitute up to 50% of all transmembrane proteins, depending on the organism, and contribute significantly to the network of interactions between different proteins in cells, including interactions via transmembrane alpha helices. They usually include one or several water-soluble domains situated at the different sides of biological membranes, for example in single-pass transmembrane receptors. Some of them are small and serve as regulatory or structure-stabilizing subunits in large multi-protein transmembrane complexes, such as photosystems or the respiratory chain. A 2013 estimate identified about 1300 single-pass membrane proteins in the human genome. Topology-based classification Bitopic proteins are classified into 4 types, depending on their transmembrane topology and location of the transmembrane hel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Domains
In molecular biology, a protein domain is a region of a protein's polypeptide chain that is self-stabilizing and that folds independently from the rest. Each domain forms a compact folded three-dimensional structure. Many proteins consist of several domains, and a domain may appear in a variety of different proteins. Molecular evolution uses domains as building blocks and these may be recombined in different arrangements to create proteins with different functions. In general, domains vary in length from between about 50 amino acids up to 250 amino acids in length. The shortest domains, such as zinc fingers, are stabilized by metal ions or disulfide bridges. Domains often form functional units, such as the calcium-binding EF hand domain of calmodulin. Because they are independently stable, domains can be "swapped" by genetic engineering between one protein and another to make chimeric proteins. Background The concept of the domain was first proposed in 1973 by Wetlaufer after ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TPP2
Tripeptidyl-peptidase 2 is an enzyme that in humans is encoded by the ''TPP2'' gene. Among other things it is heavily implicated in MHC (HLA) class-I processing, as it has both endopeptidase and exopeptidase activity. Clinical significance and genetic deficiency Biallelic deleterious variants in the TPP2 gene may result in a recessive disorder with immune deficiency, autoimmune disease and intellectual disability. Some genetic variants may result in a milder disease with sterile brain inflammation mimicking multiple sclerosis. These observations underline the fundamental role of TPP2 in cells of the immune system. References External links * The MEROPS MEROPS is an online database for peptidases (also known as proteases, proteinases and proteolytic enzymes) and their inhibitors. The classification scheme for peptidases was published by Rawlings & Barrett in 1993, and that for protein inhibit ... online database for peptidases and their inhibitorsS08.090 Further readi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PCSK9
Proprotein convertase subtilisin/kexin type 9 (PCSK9) is an enzyme encoded by the ''PCSK9'' gene in humans on chromosome 1. It is the 9th member of the proprotein convertase family of proteins that activate other proteins. Similar genes (orthologs) are found across many species. As with many proteins, PCSK9 is inactive when first synthesized, because a section of peptide chains blocks their activity; proprotein convertases remove that section to activate the enzyme. The ''PCSK9'' gene also contains one of 27 loci associated with increased risk of coronary artery disease. PCSK9 is ubiquitously expressed in many tissues and cell types. PCSK9 binds to and degrades the receptor for low-density lipoprotein particles (LDL), which typically transport 3,000 to 6,000 fat molecules (including cholesterol) per particle, within extracellular fluid. The LDL receptor (LDLR), on liver and other cell membranes, binds and initiates ingestion of LDL-particles from extracellular fluid into cells, t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PCSK7
Proprotein convertase subtilisin/kexin type 7 is an enzyme that in humans is encoded by the ''PCSK7'' gene. The protein encoded by this gene belongs to the subtilisin-like proprotein convertase family. The members of this family are proprotein convertases that process latent precursor proteins into their biologically active products. This encoded protein is a calcium-dependent serine endoprotease. It is structurally related to its family members, PACE and PACE4. This protein is concentrated in the trans- Golgi network, associated with the membranes, and is not secreted. It can process proalbumin and is thought to be responsible for the activation of HIV envelope glycoproteins gp160 and gp140. This gene has been implicated in the transcriptional regulation of housekeeping genes. Multiple alternatively spliced transcripts are described for this gene but their full length nature is not yet known. Downstream of this gene's map location at 11q23-q24, nucleotides that match part of thi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PCSK6
Proprotein convertase subtilisin/kexin type 6 is an protease that in humans is encoded by the ''PCSK6'' gene which is located in chromosome 15. Pcsk6 is a calcium-dependent serine endoprotease that catalyzes the post-translational modification of precursor proteins from its ‘latent’ form to the cleaved ‘active’ form. Active Pcsk6 has been reported to process substrates such as transforming growth factor β, pro-albumin, von Willebrand factor, and corin. Clinically, Pcsk6 is suggested to play a role in left/right asymmetry, structural asymmetry of the brain, handedness, tumor progression, hemostasis, and cardiovascular diseases. Function The protein encoded by this gene belongs to the subtilisin-like proprotein convertase family. The members of this family are proprotein convertases that process latent precursor proteins into their biologically active products. This encoded protein is a calcium-dependent serine endoprotease that can cleave precursor protein at their paire ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PCSK5
Proprotein convertase subtilisin/kexin type 5 is an enzyme that in humans is encoded by the ''PCSK5'' gene, found in chromosome 9q21.3 Two alternatively spliced transcripts are described for this gene but only one has its full length nature known. Function The protein encoded by this gene belongs to the subtilisin-like proprotein convertase family. The members of this family are proprotein convertases that process latent precursor proteins into their biologically active products. This encoded protein mediates posttranslational endoproteolytic processing for several integrin alpha subunits. It is thought to process prorenin, pro-membrane type-1 matrix metalloproteinase and HIV-1 glycoprotein gp160. Clinical significance Mutations in this gene have been associated with Currarino syndrome Currarino syndrome is an inherited congenital disorder where either the sacrum (the fused vertebrae forming the back of the pelvis) is not formed properly, or there is a mass in the presa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PCSK4
Proprotein convertase subtilisin/kexin type 4 is an enzyme that in humans is encoded by the ''PCSK4'' gene In biology, the word gene (from , ; "...Wilhelm Johannsen coined the word gene to describe the Mendelian units of heredity..." meaning ''generation'' or ''birth'' or ''gender'') can have several different meanings. The Mendelian gene is a ba .... References Further reading * * * * * * * * * * * {{gene-19-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PCSK2
Proprotein convertase 2 (PC2) also known as prohormone convertase 2 or neuroendocrine convertase 2 (NEC2) is a serine protease and proprotein convertase PC2, like proprotein convertase 1 (PC1), is an enzyme responsible for the first step in the maturation of many neuroendocrine peptides from their precursors, such as the conversion of proinsulin to insulin intermediates. To generate the bioactive form of insulin (and many other peptides), a second step involving the removal of C-terminal basic residues is required; this step is mediated by carboxypeptidases E and/or D. PC2 plays only a minor role in the first step of insulin biosynthesis, but a greater role in the first step of glucagon biosynthesis compared to PC1. PC2 binds to the neuroendocrine protein named 7B2, and if this protein is not present, proPC2 cannot become enzymatically active. 7B2 accomplishes this by preventing the aggregation of proPC2 to inactivatable forms. The C-terminal domain of 7B2 also inhibits PC2 acti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PCSK1
Proprotein convertase 1, also known as prohormone convertase, prohormone convertase 3, or neuroendocrine convertase 1 and often abbreviated as PC1/3 is an enzyme that in humans is encoded by the ''PCSK1'' gene. PCSK1 and PCSK2 differentially cleave proopiomelanocortin and they act together to process proinsulin and proglucagon in pancreatic islets. Function PC1/3 is an enzyme that performs the proteolytic cleavage of prohormones to their intermediate (or sometimes completely cleaved) forms. It is present only in neuroendocrine cells such as brain, pituitary and adrenal, and most often cleaves after a pair of basic residues within prohormones but can occasionally cleave after a single arginine. It binds to a protein known as proSAAS, which also represents its endogenous inhibitor. PC1 is synthesized as a 99 kDa proform quickly converted to an 87 kDa major active form, which itself is nearly completely cleaved to a 66 kDa active form within neuroendocrine cells. Proprotein conv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |