|
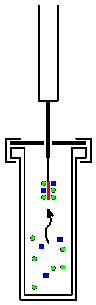
Solid Phase Microextraction
Solid phase microextraction, or SPME, is a solid phase extraction sampling technique that involves the use of a fiber coated with an extracting phase, that can be a liquid ( polymer) or a solid ( sorbent), which extracts different kinds of analytes (including both volatile and non-volatile) from different kinds of media, that can be in liquid or gas phase. The quantity of analyte extracted by the fibre is proportional to its concentration in the sample as long as equilibrium is reached or, in case of short time pre-equilibrium, with help of convection or agitation. Analysis After extraction, the SPME fiber is transferred to the injection port of separating instruments, such as a gas chromatography and mass spectrometry Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a ''mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is use ..., where d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solid Phase Extraction
Solid-phase extraction (SPE) is an extractive technique by which compounds that are dissolved or suspended in a liquid mixture are separated from other compounds in the mixture according to their physical and chemical properties. Analytical laboratories use solid phase extraction to concentrate and purify samples for analysis. Solid phase extraction can be used to isolate analytes of interest from a wide variety of matrices, including urine, blood, water, beverages, soil, and animal tissue. SPE uses the affinity of solutes dissolved or suspended in a liquid (known as the mobile phase) for a solid through which the sample is passed (known as the stationary phase) to separate a mixture into desired and undesired components. The result is that either the desired analytes of interest or undesired impurities in the sample are retained on the stationary phase. The portion that passes through the stationary phase is collected or discarded, depending on whether it contains the desired ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymer
A polymer (; Greek ''poly-'', "many" + '' -mer'', "part") is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties including toughness, high elasticity, viscoelasticity, and a tendency to form amorphous and semicrystalline structures rather than crystals. The term "polymer" derives from the Greek word πολύς (''polus'', meaning "many, much") and μέρος (''meros'', mean ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sorbent
A sorbent is a material used to absorb or adsorb liquids or gases. Examples include: *A material similar to molecular sieve material, which acts by adsorption (attracting molecules to its surface). It has a large internal surface area and good thermal conductivity. It is typically supplied in pellets of 1 mm to 2 mm diameter and roughly 5 mm length or as grains of the order 1 mm. Occasionally as beads up to 5 mm diameter. They are typically made from aluminium oxide with a porous structure. *Materials used to absorb other materials due to their high affinity for doing so. Examples include: **In composting, dry (brown, high-carbon) materials absorb many odoriferous chemicals, and these chemicals help to decompose these sorbents. **A sponge absorbs many times its own weight in water. **A polypropylene fiber mat may be used to absorb oil. **A cellulose fiber product may be used to absorb oil. **The granular gel material in a baby diaper will absorb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Analyte
An analyte, component (in clinical chemistry), or chemical species is a substance or chemical constituent that is of interest in an analytical procedure. The purest substances are referred to as analytes, such as 24 karat gold, NaCl, water, etc. In reality, no substance has been found to be 100% pure in its quality, so a substance that is found to be most pure (for some metals, 99% after electrolysis) is called an analyte. See also *Analytical chemistry * Immunoassay *Magnetic immunoassay Magnetic immunoassay (MIA) is a type of diagnostic immunoassay using magnetic beads as labels in lieu of conventional enzymes (ELISA), radioisotopes ( RIA) or fluorescent moieties ( fluorescent immunoassays) to detect a specified analyte. MIA invo ... References Analytical chemistry {{Analytical-chemistry-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Volatility (chemistry)
In chemistry, volatility is a material quality which describes how readily a substance vaporizes. At a given temperature and pressure, a substance with high volatility is more likely to exist as a vapour, while a substance with low volatility is more likely to be a liquid or solid. Volatility can also describe the tendency of a vapor to condense into a liquid or solid; less volatile substances will more readily condense from a vapor than highly volatile ones. Differences in volatility can be observed by comparing how fast substances within a group evaporate (or sublimate in the case of solids) when exposed to the atmosphere. A highly volatile substance such as rubbing alcohol (isopropyl alcohol) will quickly evaporate, while a substance with low volatility such as vegetable oil will remain condensed. In general, solids are much less volatile than liquids, but there are some exceptions. Solids that sublimate (change directly from solid to vapor) such as dry ice (solid carbon dio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Equilibrium
In a chemical reaction, chemical equilibrium is the state in which both the reactants and products are present in concentrations which have no further tendency to change with time, so that there is no observable change in the properties of the system. This state results when the forward reaction proceeds at the same rate as the reverse reaction. The reaction rates of the forward and backward reactions are generally not zero, but they are equal. Thus, there are no net changes in the concentrations of the reactants and products. Such a state is known as dynamic equilibrium. Historical introduction The concept of chemical equilibrium was developed in 1803, after Berthollet found that some chemical reactions are reversible. For any reaction mixture to exist at equilibrium, the rates of the forward and backward (reverse) reactions must be equal. In the following chemical equation, arrows point both ways to indicate equilibrium. A and B are reactant chemical species, S and T a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Convection
Convection is single or multiphase fluid flow that occurs spontaneously due to the combined effects of material property heterogeneity and body forces on a fluid, most commonly density and gravity (see buoyancy). When the cause of the convection is unspecified, convection due to the effects of thermal expansion and buoyancy can be assumed. Convection may also take place in soft solids or mixtures where particles can flow. Convective flow may be transient (such as when a multiphase mixture of oil and water separates) or steady state (see Convection cell). The convection may be due to gravitational, electromagnetic or fictitious body forces. Heat transfer by natural convection plays a role in the structure of Earth's atmosphere, its oceans, and its mantle. Discrete convective cells in the atmosphere can be identified by clouds, with stronger convection resulting in thunderstorms. Natural convection also plays a role in stellar physics. Convection is often cate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gas Chromatography
Gas chromatography (GC) is a common type of chromatography used in analytical chemistry for separating and analyzing compounds that can be vaporized without decomposition. Typical uses of GC include testing the purity of a particular substance, or separating the different components of a mixture. In preparative chromatography, GC can be used to prepare pure compounds from a mixture. Gas chromatography is also sometimes known as vapor-phase chromatography (VPC), or gas–liquid partition chromatography (GLPC). These alternative names, as well as their respective abbreviations, are frequently used in scientific literature. Gas chromatography is the process of separating compounds in a mixture by injecting a gaseous or liquid sample into a mobile phase, typically called the carrier gas, and passing the gas through a stationary phase. The mobile phase is usually an inert gas or an unreactive gas such as helium, argon, nitrogen or hydrogen. The stationary phase is a microscopic lay ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mass Spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a '' mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is used in many different fields and is applied to pure samples as well as complex mixtures. A mass spectrum is a type of plot of the ion signal as a function of the mass-to-charge ratio. These spectra are used to determine the elemental or isotopic signature of a sample, the masses of particles and of molecules, and to elucidate the chemical identity or structure of molecules and other chemical compounds. In a typical MS procedure, a sample, which may be solid, liquid, or gaseous, is ionized, for example by bombarding it with a beam of electrons. This may cause some of the sample's molecules to break up into positively charged fragments or simply become positively charged without fragmenting. These ions (fragments) are then separated acco ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Desorption
Desorption is the physical process where a previously adsorbed substance is released from a surface. This happens when a molecule gains enough energy to overcome the activation barrier of the bounding energy that keeps it in the surface. There are a lot of different types of desorption, depending on the mechanism that separates the adsorbate from the substrate; therefore there is no one equation that describes the process. Note that desorption is the opposite of adsorption, which differs from absorption because it refers to substances being stuck to the surface, as opposed to being absorbed into the bulk. Desorption can occur after a reaction between a catalyst and an adsorbed compound; or during stripping or chromatography which are types of separation processes. Desorption mechanisms Depending on the nature of the adsorbent-to-surface bond, there are a multitude of mechanisms for desorption. The surface bond of a sorbant can be cleaved thermally, through chemical reacti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polydimethylsiloxane
Polydimethylsiloxane (PDMS), also known as dimethylpolysiloxane or dimethicone, belongs to a group of polymeric organosilicon compounds that are commonly referred to as silicones. PDMS is the most widely used silicon-based organic polymer, as its versatility and properties lead to many applications. It is particularly known for its unusual rheological (or flow) properties. PDMS is optically clear and, in general, inert, non-toxic, and non-flammable. It is one of several types of silicone oil (polymerized siloxane). Its applications range from contact lenses and medical devices to elastomers; it is also present in shampoos (as it makes hair shiny and slippery), food ( antifoaming agent), caulk, lubricants and heat-resistant tiles. Structure The chemical formula of PDMS is , where ''n'' is the number of repeating monomer units.Mark, J. E.; Allcock, H. R.; West, R. “Inorganic Polymers” Prentice Hall, Englewood, NJ: 1992. . Industrial synthesis can begin from dimethy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |






.jpg)
