|
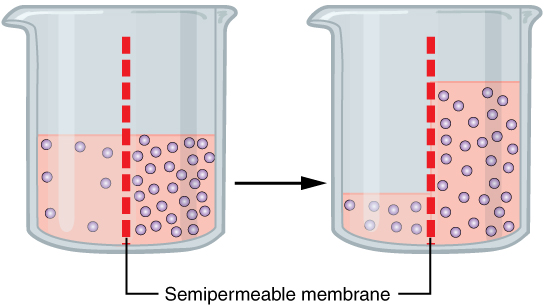
Semipermeable
Semipermeable membrane is a type of biological or synthetic, polymeric membrane that will allow certain molecules or ions to pass through it by osmosis. The rate of passage depends on the pressure, concentration, and temperature of the molecules or solutes on either side, as well as the permeability of the membrane to each solute. Depending on the membrane and the solute, permeability may depend on solute size, solubility, properties, or chemistry. How the membrane is constructed to be selective in its permeability will determine the rate and the permeability. Many natural and synthetic materials which are rather thick are also semipermeable. One example of this is the thin film on the inside of the egg. Biological membranes are selectively permeable, with the passage of molecules controlled by facilitated diffusion, passive transport or active transport regulated by proteins embedded in the membrane. Biological membranes An example of a biological semi-permeable membrane is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Semipermeable Membrane (svg)
Semipermeable membrane is a type of biological or synthetic, polymeric membrane that will allow certain molecules or ions to pass through it by osmosis. The rate of passage depends on the pressure, concentration, and temperature of the molecules or solutes on either side, as well as the permeability of the membrane to each solute. Depending on the membrane and the solute, permeability may depend on solute size, solubility, properties, or chemistry. How the membrane is constructed to be selective in its permeability will determine the rate and the permeability. Many natural and synthetic materials which are rather thick are also semipermeable. One example of this is the thin film on the inside of the egg. Biological membranes are selectively permeable, with the passage of molecules controlled by facilitated diffusion, passive transport or active transport regulated by proteins embedded in the membrane. Biological membranes An example of a biological semi-permeable membrane is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Osmosis
Osmosis (, ) is the spontaneous net movement or diffusion of solvent molecules through a selectively-permeable membrane from a region of high water potential (region of lower solute concentration) to a region of low water potential (region of higher solute concentration), in the direction that tends to equalize the solute concentrations on the two sides. It may also be used to describe a physical process in which any solvent moves across a selectively permeable membrane (permeable to the solvent, but not the solute) separating two solutions of different concentrations. Osmosis can be made to do work. Osmotic pressure is defined as the external pressure required to be applied so that there is no net movement of solvent across the membrane. Osmotic pressure is a colligative property, meaning that the osmotic pressure depends on the molar concentration of the solute but not on its identity. Osmosis is a vital process in biological systems, as biological membranes are semip ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reverse Osmosis
Reverse osmosis (RO) is a water purification process that uses a partially permeable membrane to separate ions, unwanted molecules and larger particles from drinking water. In reverse osmosis, an applied pressure is used to overcome osmotic pressure, a colligative property that is driven by chemical potential differences of the solvent, a thermodynamic parameter. Reverse osmosis can remove many types of dissolved and suspended chemical species as well as biological ones (principally bacteria) from water, and is used in both industrial processes and the production of potable water. The result is that the solute is retained on the pressurized side of the membrane and the pure solvent is allowed to pass to the other side. To be "selective", this membrane should not allow large molecules or ions through the pores (holes), but should allow smaller components of the solution (such as solvent molecules, e.g., water, H2O) to pass freely. In the normal osmosis process, the solvent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plasma Membrane
The cell membrane (also known as the plasma membrane (PM) or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of all cells from the outside environment (the extracellular space). The cell membrane consists of a lipid bilayer, made up of two layers of phospholipids with cholesterols (a lipid component) interspersed between them, maintaining appropriate membrane fluidity at various temperatures. The membrane also contains membrane proteins, including integral proteins that span the membrane and serve as membrane transporters, and peripheral proteins that loosely attach to the outer (peripheral) side of the cell membrane, acting as enzymes to facilitate interaction with the cell's environment. Glycolipids embedded in the outer lipid layer serve a similar purpose. The cell membrane controls the movement of substances in and out of cells and organelles, being selectively permeable to io ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Osmotic Pressure
Osmotic pressure is the minimum pressure which needs to be applied to a solution to prevent the inward flow of its pure solvent across a semipermeable membrane. It is also defined as the measure of the tendency of a solution to take in a pure solvent by osmosis. Potential osmotic pressure is the maximum osmotic pressure that could develop in a solution if it were separated from its pure solvent by a semipermeable membrane. Osmosis occurs when two solutions containing different concentrations of solute are separated by a selectively permeable membrane. Solvent molecules pass preferentially through the membrane from the low-concentration solution to the solution with higher solute concentration. The transfer of solvent molecules will continue until equilibrium is attained. Theory and measurement Jacobus van 't Hoff found a quantitative relationship between osmotic pressure and solute concentration, expressed in the following equation: :\Pi = icRT where \Pi is osmotic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cell (biology)
The cell is the basic structural and functional unit of life forms. Every cell consists of a cytoplasm enclosed within a membrane, and contains many biomolecules such as proteins, DNA and RNA, as well as many small molecules of nutrients and metabolites.Cell Movements and the Shaping of the Vertebrate Body in Chapter 21 of Molecular Biology of the Cell '' fourth edition, edited by Bruce Alberts (2002) published by Garland Science. The Alberts text discusses how the "cellular building blocks" move to shape developing s. It is also common ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thin-film Composite Membrane
Thin-film composite membranes (TFC or TFM) are semipermeable membranes manufactured principally for use in water purification or water desalination systems. They also have use in chemical applications such as batteries and fuel cells. A TFC membrane can be considered as a molecular sieve constructed in the form of a film from two or more layered materials. TFC membranes are commonly classified as nanofiltration (NF) and reverse osmosis (RO) membranes. Both types are typically made out of a thin polyamide layer (<200 nm) deposited on top of a or porous layer (about 50 microns) on top of a |
Membrane
A membrane is a selective barrier; it allows some things to pass through but stops others. Such things may be molecules, ions, or other small particles. Membranes can be generally classified into synthetic membranes and biological membranes. Biological membranes include cell membranes (outer coverings of cells or organelles that allow passage of certain constituents); nuclear membranes, which cover a cell nucleus; and tissue membranes, such as mucosae and serosae. Synthetic membranes are made by humans for use in laboratories and industry (such as chemical plants). This concept of a membrane has been known since the eighteenth century but was used little outside of the laboratory until the end of World War II. Drinking water supplies in Europe had been compromised by the war and membrane filters were used to test for water safety. However, due to the lack of reliability, slow operation, reduced selectivity and elevated costs, membranes were not widely exploited. The fir ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fatty Acid
In chemistry, particularly in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, from 4 to 28. Fatty acids are a major component of the lipids (up to 70% by weight) in some species such as microalgae but in some other organisms are not found in their standalone form, but instead exist as three main classes of esters: triglycerides, phospholipids, and cholesteryl esters. In any of these forms, fatty acids are both important dietary sources of fuel for animals and important structural components for cells. History The concept of fatty acid (''acide gras'') was introduced in 1813 by Michel Eugène Chevreul, though he initially used some variant terms: ''graisse acide'' and ''acide huileux'' ("acid fat" and "oily acid"). Types of fatty acids Fatty acids are classified in many ways: by length, by saturation vs unsat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Synthesis
As a topic of chemistry, chemical synthesis (or combination) is the artificial execution of chemical reactions to obtain one or several products. This occurs by physical and chemical manipulations usually involving one or more reactions. In modern laboratory uses, the process is reproducible and reliable. A chemical synthesis involves one or more compounds (known as ''reagents'' or ''reactants'') that will experience a transformation when subjected to certain conditions. Various reaction types can be applied to formulate a desired product. This requires mixing the compounds in a reaction vessel, such as a chemical reactor or a simple round-bottom flask. Many reactions require some form of processing ("work-up") or purification procedure to isolate the final product. The amount produced by chemical synthesis is known as the ''reaction yield''. Typically, yields are expressed as a mass in grams (in a laboratory setting) or as a percentage of the total theoretical quanti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bulk Flow
Mass flow, also known as mass transfer and bulk flow, is the movement of fluids down a pressure or temperature gradient,Moyes & Schulte (2008). Principles of Animal Physiology. Pearson Benjamin Cummings. San Francisco, California particularly in the life sciences. As such, mass flow is a subject of study in both fluid dynamics and biology. Examples of mass flow include blood circulation and transport of water in vascular plant tissues. Mass flow is not to be confused with diffusion which depends on concentration gradients within a medium rather than pressure gradients of the medium itself. Plant biology In general, bulk flow in plant biology typically refers to the movement of water from the soil up through the plant to the leaf tissue through xylem, but can also be applied to the transport of larger solutes (e.g. sucrose) through the phloem. Xylem According to cohesion-tension theory, water transport in xylem relies upon the cohesion of water molecules to each other and adhesio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
G Protein-coupled Receptor
G protein-coupled receptors (GPCRs), also known as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptors, and G protein-linked receptors (GPLR), form a large group of evolutionarily-related proteins that are cell surface receptors that detect molecules outside the cell and activate cellular responses. Coupling with G proteins, they are called seven-transmembrane receptors because they pass through the cell membrane seven times. Text was copied from this source, which is available under Attribution 2.5 Generic (CC BY 2.5) license. Ligands can bind either to extracellular N-terminus and loops (e.g. glutamate receptors) or to the binding site within transmembrane helices (Rhodopsin-like family). They are all activated by agonists although a spontaneous auto-activation of an empty receptor can also be observed. G protein-coupled receptors are found only in eukaryotes, including yeast, choanoflagellates, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |