|
Sapanisertib
Sapanisertib (also known as MLN0128, INK128 and TAK-228) is an experimental small molecule inhibitor of mTOR which is administered orally. It targets both mTORC1 and mTORC2. Developed by Millennium Pharmaceuticals, and is in phase II clinical trials for breast cancer, endometrial cancer, glioblastoma, renal cell carcinoma, and thyroid cancer. The drug has been well tolerated by patients with advanced solid tumours in Phase I trials The phases of clinical research are the stages in which scientists conduct experiments with a health intervention to obtain sufficient evidence for a process considered effective as a medical treatment. For drug development, the clinical phases .... References {{reflist Experimental cancer drugs Benzoxazoles Isopropyl compounds Amines ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MTOR Inhibitors
mTOR inhibitors are a drug class, class of drugs that inhibit the mechanistic target of rapamycin (mTOR), which is a serine/threonine-specific protein kinase that belongs to the family of Phosphoinositide 3-kinase, phosphatidylinositol-3 kinase (PI3K) related kinases (PIKKs). mTOR regulates cellular metabolism, growth, and proliferation by forming and signaling through two protein complexes, mTORC1 and mTORC2. The most established mTOR inhibitors are so-called rapalogs (rapamycin and its analogs), which have shown tumor responses in clinical trials against various tumor types. History The discovery of mTOR was made a few decades ago while investigating the mechanism of action of its enzyme inhibitor, inhibitor, rapamycin. Rapamycin was first discovered in 1975 in a soil sample from Easter Island of Oceania, South Pacific, also known as Rapa Nui, from where its name is derived. Rapamycin is a macrolide, produced by the microorganism ''Streptomyces hygroscopicus'' and showed antifun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Experimental Drug
An experimental drug is a medicinal product (a drug or vaccine) that has not yet received approval from governmental regulatory authorities for routine use in human or veterinary medicine. A medicinal product may be approved for use in one disease or condition but still be considered experimental for other diseases or conditions. In 2018 the United States of America signed the legislation "Right to Try", this allows individuals who fit into the criteria to try experimental drugs that are not yet deemed safe. In the United States, the body responsible for approval is the U.S. Food and Drug Administration (FDA), which must grant the substance Investigational New Drug (IND) status before it can be tested in human clinical trials. IND status requires the drug's sponsor to submit an IND application that includes data from laboratory and animal testing for safety and efficacy. A drug that is made from a living organism or its products undergoes the same approval process but is called a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MTORC1
mTORC1, also known as mammalian target of rapamycin complex 1 or mechanistic target of rapamycin complex 1, is a protein complex that functions as a nutrient/energy/redox sensor and controls protein synthesis. mTOR Complex 1 (mTORC1) is composed of the mammalian target of rapamycin, mTOR protein complex, RPTOR, regulatory-associated protein of mTOR (commonly known as raptor), mammalian lethal with SEC13 protein 8 (MLST8), AKT1S1, PRAS40 and DEPTOR. This complex embodies the classic functions of mTOR, namely as a nutrient/energy/redox sensor and controller of protein synthesis. The activity of this complex is regulated by rapamycin, insulin, growth factors, phosphatidic acid, certain amino acids and their derivatives (e.g., leucine, -leucine and β-hydroxy β-methylbutyric acid), mechanical stimuli, and oxidative stress. Recently it has been also demonstrated that cellular bicarbonate metabolism can be regulated by mTORC1 signaling. The role of mTORC1 is to activate translation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Millennium Pharmaceuticals
Takeda Oncology (originally Millennium Pharmaceuticals) is a biopharmaceutical company based in Cambridge, Massachusetts. It is a fully owned subsidiary of Takeda Pharmaceutical. Takeda Oncology's research, development and commercialization activities focused in two therapeutic areas: oncology and inflammation to develop a line of new product candidates. It was one of the first companies to systematically search for genes linked to disease, although none of the drugs which it is marketing or has in clinical trial, with one partial exception, have been the results of that research. It is particularly known for bringing bortezomib (marketed as Velcade) through clinical trials to approval for treatment of patients with multiple myeloma by the U.S. FDA, but has a growing clinical development pipeline of other product candidates. On May 14, 2008, Japanese company Takeda Pharmaceutical announced the completion of its acquisition of Millennium for US$25.00 per share in cash—a deal wo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase II Clinical Trial
The phases of clinical research are the stages in which scientists conduct experiments with a health intervention to obtain sufficient evidence for a process considered effective as a medical treatment. For drug development, the clinical phases start with testing for safety in a few human subjects, then expand to many study participants (potentially tens of thousands) to determine if the treatment is effective. Clinical research is conducted on drug candidates, vaccine candidates, new medical devices, and new diagnostic assays. Summary Clinical trials testing potential medical products are commonly classified into four phases. The drug development process will normally proceed through all four phases over many years. If the drug successfully passes through Phases I, II, and III, it will usually be approved by the national regulatory authority for use in the general population. Phase IV trials are 'post-marketing' or 'surveillance' studies conducted to monitor safety over sever ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Breast Cancer
Breast cancer is cancer that develops from breast tissue. Signs of breast cancer may include a lump in the breast, a change in breast shape, dimpling of the skin, milk rejection, fluid coming from the nipple, a newly inverted nipple, or a red or scaly patch of skin. In those with distant spread of the disease, there may be bone pain, swollen lymph nodes, shortness of breath, or yellow skin. Risk factors for developing breast cancer include obesity, a lack of physical exercise, alcoholism, hormone replacement therapy during menopause, ionizing radiation, an early age at first menstruation, having children late in life or not at all, older age, having a prior history of breast cancer, and a family history of breast cancer. About 5–10% of cases are the result of a genetic predisposition inherited from a person's parents, including BRCA1 and BRCA2 among others. Breast cancer most commonly develops in cells from the lining of milk ducts and the lobules that supply these ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Endometrial Cancer
Endometrial cancer is a cancer that arises from the endometrium (the lining of the uterus or womb). It is the result of the abnormal growth of cells that have the ability to invade or spread to other parts of the body. The first sign is most often vaginal bleeding not associated with a menstrual period. Other symptoms include pain with urination, pain during sexual intercourse, or pelvic pain. Endometrial cancer occurs most commonly after menopause. Approximately 40% of cases are related to obesity. Endometrial cancer is also associated with excessive estrogen exposure, high blood pressure and diabetes. Whereas taking estrogen alone increases the risk of endometrial cancer, taking both estrogen and a progestogen in combination, as in most birth control pills, decreases the risk. Between two and five percent of cases are related to genes inherited from the parents. Endometrial cancer is sometimes loosely referred to as "uterine cancer", although it is distinct from other fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glioblastoma
Glioblastoma, previously known as glioblastoma multiforme (GBM), is one of the most aggressive types of cancer that begin within the brain. Initially, signs and symptoms of glioblastoma are nonspecific. They may include headaches, personality changes, nausea, and symptoms similar to those of a stroke. Symptoms often worsen rapidly and may progress to unconsciousness. The cause of most cases of glioblastoma is not known. Uncommon risk factors include genetic disorders, such as neurofibromatosis and Li–Fraumeni syndrome, and previous radiation therapy. Glioblastomas represent 15% of all brain tumors. They can either start from normal brain cells or develop from an existing low-grade astrocytoma. The diagnosis typically is made by a combination of a CT scan, MRI scan, and tissue biopsy. There is no known method of preventing the cancer. Treatment usually involves surgery, after which chemotherapy and radiation therapy are used. The medication temozolomide is frequently used ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Renal Cell Carcinoma
Renal cell carcinoma (RCC) is a kidney cancer that originates in the lining of the proximal convoluted tubule, a part of the very small tubes in the kidney that transport primary urine. RCC is the most common type of kidney cancer in adults, responsible for approximately 90–95% of cases. RCC occurrence shows a male predominance over women with a ratio of 1.5:1. RCC most commonly occurs between 6th and 7th decade of life. Initial treatment is most commonly either partial or complete removal of the affected kidney(s). Where the cancer has not metastasised (spread to other organs) or burrowed deeper into the tissues of the kidney, the five-year survival rate is 65–90%, but this is lowered considerably when the cancer has spread. The body is remarkably good at hiding the symptoms and as a result people with RCC often have advanced disease by the time it is discovered. The initial symptoms of RCC often include blood in the urine (occurring in 40% of affected persons at the time th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
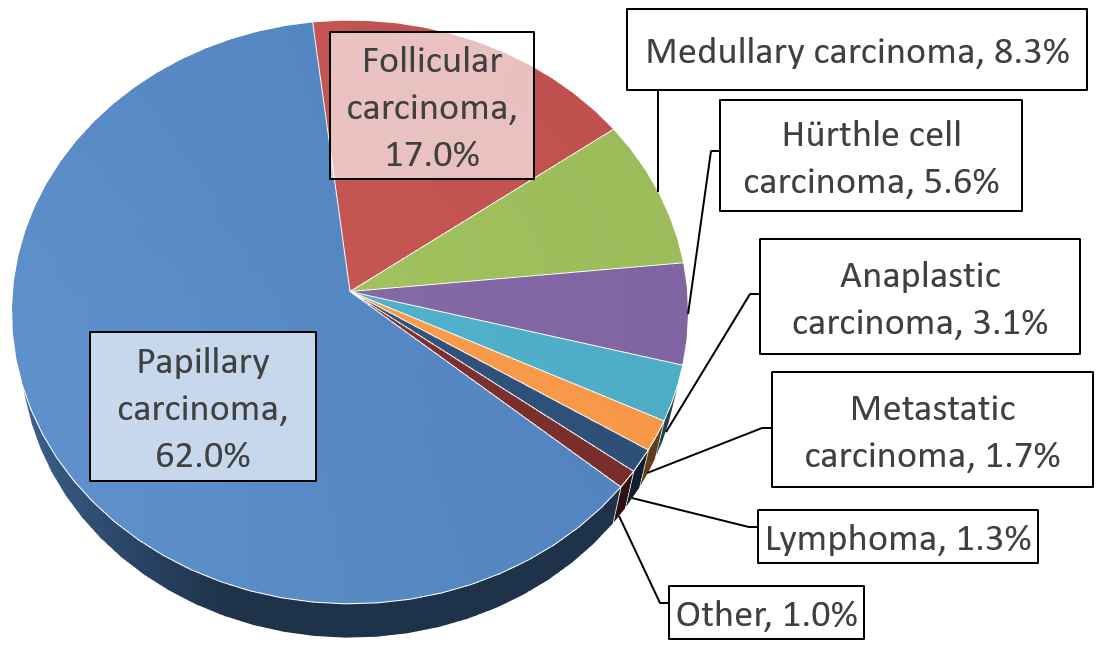
Thyroid Cancer
Thyroid cancer is cancer that develops from the tissues of the thyroid gland. It is a disease in which cells grow abnormally and have the potential to spread to other parts of the body. Symptoms can include swelling or a lump in the neck. Cancer can also occur in the thyroid after spread from other locations, in which case it is not classified as thyroid cancer. Risk factors include radiation exposure at a young age, having an enlarged thyroid, and family history. The four main types are papillary thyroid cancer, follicular thyroid cancer, medullary thyroid cancer, and anaplastic thyroid cancer. Diagnosis is often based on ultrasound and fine needle aspiration. Screening people without symptoms and at normal risk for the disease is not recommended as of 2017. Treatment options may include surgery, radiation therapy including radioactive iodine, chemotherapy, thyroid hormone, targeted therapy, and watchful waiting. Surgery may involve removing part or all of the thyroid. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tolerability
Tolerability refers to the degree to which overt adverse effects of a drug can be tolerated by a patient. Tolerability of a particular drug can be discussed in a general sense, or it can be a quantifiable measurement as part of a clinical study. Usually, it is measured by the rate of "dropouts", or patients that forfeit participation in a study due to extreme adverse effects. Tolerability, however, is often relative to the severity of the medical condition a drug is designed to treat. For instance, cancer patients may tolerate significant pain or discomfort during a chemotherapeutic study with the hope of prolonging survival or finding a cure, whereas patients experiencing a benign condition, such as a headache, are less likely to. As an example, tricyclic antidepressants (TCAs) are very poorly tolerated and often produce severe side effects including sedation, orthostatic hypotension, and anticholinergic effects, whereas newer antidepressants have far fewer adverse effects and are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |




_Nephrectomy.jpg)