|
Rubidium-85
Rubidium (37Rb) has 36 isotopes, with naturally occurring rubidium being composed of just two isotopes; 85Rb (72.2%) and the radioactive 87Rb (27.8%). Normal mixes of rubidium are radioactive enough to fog photographic film in approximately 30 to 60 days. 87Rb has a half-life of . It readily substitutes for potassium in minerals, and is therefore fairly widespread. 87Rb has been used extensively in dating rocks; 87Rb decays to stable strontium-87 by emission of a beta particle (an electron ejected from the nucleus). During fractional crystallization, Sr tends to become concentrated in plagioclase, leaving Rb in the liquid phase. Hence, the Rb/Sr ratio in residual magma may increase over time, resulting in rocks with increasing Rb/Sr ratios with increasing differentiation. The highest ratios (10 or higher) occur in pegmatites. If the initial amount of Sr is known or can be extrapolated, the age can be determined by measurement of the Rb and Sr concentrations and the 87Sr/8 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
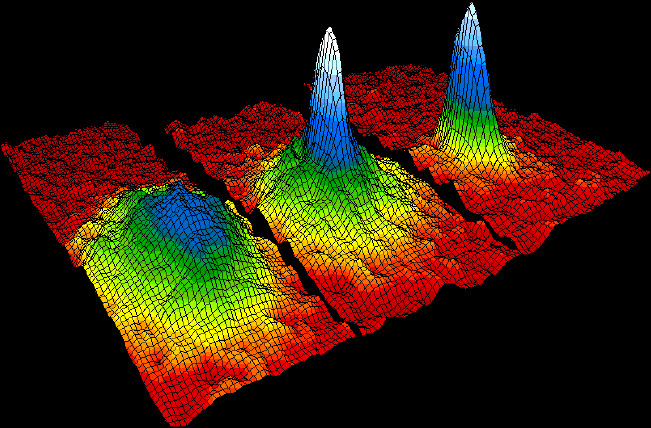
Bose–Einstein Condensate
In condensed matter physics, a Bose–Einstein condensate (BEC) is a state of matter that is typically formed when a gas of bosons at very low densities is cooled to temperatures very close to absolute zero (−273.15 °C or −459.67 °F). Under such conditions, a large fraction of bosons occupy the lowest quantum state, at which point microscopic quantum mechanical phenomena, particularly wavefunction interference, become apparent macroscopically. A BEC is formed by cooling a gas of extremely low density (about 100,000 times less dense than normal air) to ultra-low temperatures. This state was first predicted, generally, in 1924–1925 by Albert Einstein following and crediting a pioneering paper by Satyendra Nath Bose on the new field now known as quantum statistics. In 1995, the Bose-Einstein condensate was created by Eric Cornell and Carl Wieman of the University of Colorado at Boulder using rubidium atoms; later that year, Wolfgang Ketterle of MIT produc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |