|
Right To Withdraw
The right to withdraw is a concept in clinical research ethics that a study participant in a clinical trial has a right to end participation in that trial at will. According to ICH GCP guidelines, a person can withdraw from the research at any point in time and the participant is not required to reveal the reason for discontinuation. Children in research When children participate in clinical research their parents or guardians must give assent for them to participate, but ethics dictate that even in this case it is best to get the consent of the research subject. Studies have shown that children participating in research have little understanding of the right to withdraw when they are presented with the option. Biobanks Withdrawal from participating in biobank A biobank is a type of biorepository that stores biological samples (usually human) for use in research. Biobanks have become an important resource in medical research, supporting many types of contemporary research like ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Research Ethics
Clinical research ethics are the set of relevant ethics considered in the conduct of a clinical trial in the field of clinical research. It borrows from the broader fields of research ethics and medical ethics. Governance Most directly a local institutional review board oversees the clinical research ethics of any given clinical trial. The institutional review board understands and acts according to local and national law. Each countries national law is guided by international principles, such as the Belmont Report's directive that all study participants have a right to "respect for persons", "beneficence", and "justice" when participating in clinical research. Study participant rights Participants in clinical research have rights which they should expect, including the following: *right to informed consent *shared decision-making *privacy for research participants *return of results *right to withdraw Vulnerable populations There is a range of autonomy which study participants ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Trial
Clinical trials are prospective biomedical or behavioral research studies on human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments (such as novel vaccines, drugs, dietary choices, dietary supplements, and medical devices) and known interventions that warrant further study and comparison. Clinical trials generate data on dosage, safety and efficacy. They are conducted only after they have received health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted. Depending on product type and development stage, investigators initially enroll volunteers or patients into small pilot studies, and subsequently conduct progressively larger scale comparative studies. Clinical trials can vary i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
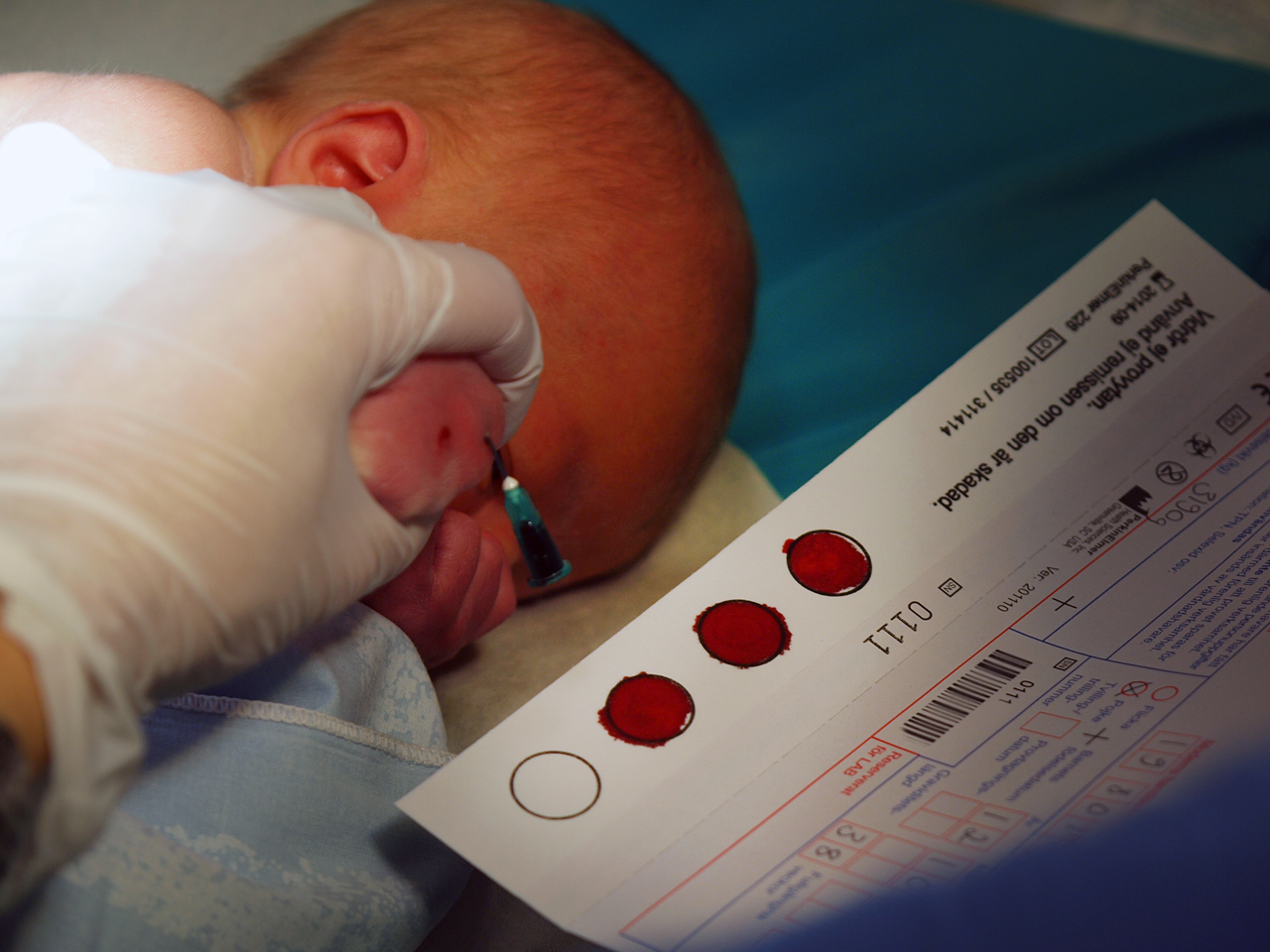
Biobank
A biobank is a type of biorepository that stores biological samples (usually human) for use in research. Biobanks have become an important resource in medical research, supporting many types of contemporary research like genomics and personalized medicine. Biobanks can give researchers access to data representing a large number of people. Samples in biobanks and the data derived from those samples can often be used by multiple researchers for cross purpose research studies. For example, many diseases are associated with single-nucleotide polymorphisms. Genome-wide association studies using data from tens or hundreds of thousands of individuals can identify these genetic associations as potential disease biomarkers. Many researchers struggled to acquire sufficient samples prior to the advent of biobanks. Biobanks have provoked questions on privacy, research ethics, and medical ethics. Viewpoints on what constitutes appropriate biobank ethics diverge. However, a consensus has bee ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
De-identification
De-identification is the process used to prevent someone's personal identity from being revealed. For example, data produced during human subject research might be de-identified to preserve the privacy of research participants. Biological data may be de-identified in order to comply with HIPAA regulations that define and stipulate patient privacy laws. When applied to metadata or general data about identification, the process is also known as data anonymization. Common strategies include deleting or masking personal identifiers, such as personal name, and suppressing or generalizing quasi-identifiers, such as date of birth. The reverse process of using de-identified data to identify individuals is known as data re-identification. Successful re-identifications cast doubt on de-identification's effectiveness. A systematic review of fourteen distinct re-identification attacks found "a high re-identification rate €¦dominated by small-scale studies on data that was not de-identifi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Privacy For Research Participants
Privacy for research participants is a concept in research ethics which states that a person in human subject research has a right to privacy when participating in research. Some typical scenarios this would apply to include, or example, a surveyor doing social research conducts an interview with a participant, or a medical researcher in a clinical trial asks for a blood sample from a participant to see if there is a relationship between something which can be measured in blood and a person's health. In both cases, the ideal outcome is that any participant can join the study and neither the researcher nor the study design nor the publication of the study results would ever identify any participant in the study. Thus, the privacy rights of these individuals can be preserved. Privacy for medical research participants is protected by several procedures such as informed consent, compliance with medical privacy laws, and transparency in how patient data is accumulated and analyzed. Peo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Research Ethics
Research is " creative and systematic work undertaken to increase the stock of knowledge". It involves the collection, organization and analysis of evidence to increase understanding of a topic, characterized by a particular attentiveness to controlling sources of bias and error. These activities are characterized by accounting and controlling for biases. A research project may be an expansion on past work in the field. To test the validity of instruments, procedures, or experiments, research may replicate elements of prior projects or the project as a whole. The primary purposes of basic research (as opposed to applied research) are documentation, discovery, interpretation, and the research and development (R&D) of methods and systems for the advancement of human knowledge. Approaches to research depend on epistemologies, which vary considerably both within and between humanities and sciences. There are several forms of research: scientific, humanities, artistic, econ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.jpg)