|
Ricin
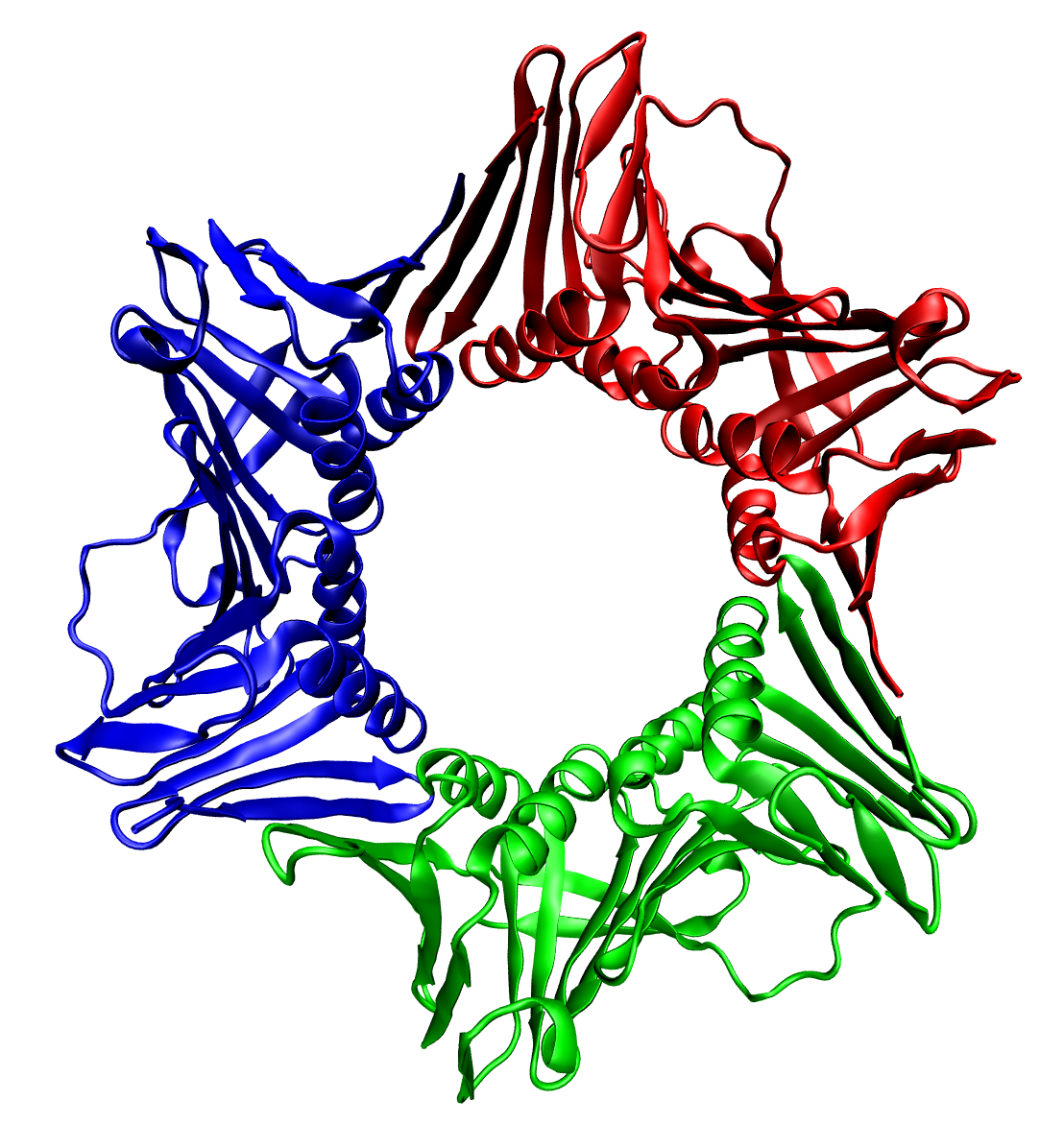
Ricin ( ) is a lectin (a carbohydrate-binding protein) and a highly potent toxin produced in the seeds of the castor oil plant, ''Ricinus communis''. The median lethal dose (LD50) of ricin for mice is around 22 micrograms per kilogram of body weight via intraperitoneal injection. Oral exposure to ricin is far less toxic. An estimated lethal oral dose in humans is approximately 1 milligram per kilogram of body weight. Ricin was first described by Peter Hermann Stillmark, the founder of lectinology. Biochemistry Ricin is classified as a type 2 ribosome-inactivating protein (RIP). Whereas type 1 RIPs are composed of a single protein chain that possesses catalytic activity, type 2 RIPs, also known as holotoxins, are composed of two different protein chains that form a heterodimeric complex. Type 2 RIPs consist of an A chain that is functionally equivalent to a type 1 RIP, covalently connected by a single disulfide bond to a B chain that is catalytically inactive, but serve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Abrin
Abrin is an extremely toxic toxalbumin found in the seeds of the rosary pea (or jequirity pea), ''Abrus precatorius''. It has a median lethal dose of 0.7 micrograms per kilogram of body mass when given to mice intravenously (approximately 31.4 times more toxic than ricin, being 22 micrograms per kilogram). The median toxic dose for humans ranges from 10 to 1000 micrograms per kilogram when ingested and is 3.3 micrograms per kilogram when inhaled. Abrin is a ribosome inhibiting protein like ricin, a toxin which can be found in the seeds of the castor oil plant, and pulchellin, a toxin which can be found in the seeds of ''Abrus pulchellus''. Abrin is classed as a " select agent" under U.S. law. Occurrence Abrin is only formed in nature by the rosary pea. The brightly coloured seeds of this plant contain about 0.08% of abrin. The toxin is found within the seeds and its release is prevented by the seed coat. If the seed coat is injured or destroyed (by chewing, for e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Castor Oil Plant
''Ricinus communis'', the castor bean or castor oil plant, is a species of perennial flowering plant in the spurge family, Euphorbiaceae. It is the sole species in the monotypic genus, ''Ricinus'', and subtribe, Ricininae. The evolution of castor and its relation to other species are currently being studied using modern genetic tools. It reproduces with a mixed pollination system which favors selfing by geitonogamy but at the same time can be an out-crosser by anemophily (wind pollination) or entomophily (insect pollination). Its seed is the castor bean, which, despite its name, is not a bean (that is, the seed of many Fabaceae). Castor is indigenous to the southeastern Mediterranean Basin, Eastern Africa, and India, but is widespread throughout tropical regions (and widely grown elsewhere as an ornamental plant). Castor seed is the source of castor oil, which has a wide variety of uses. The seeds contain between 40% and 60% oil that is rich in triglycerides, mainly ricino ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lectin
Lectins are carbohydrate-binding proteins that are highly specific for sugar groups that are part of other molecules, so cause agglutination of particular cells or precipitation of glycoconjugates and polysaccharides. Lectins have a role in recognition at the cellular and molecular level and play numerous roles in biological recognition phenomena involving cells, carbohydrates, and proteins. Lectins also mediate attachment and binding of bacteria, viruses, and fungi to their intended targets. Lectins are ubiquitous in nature and are found in many foods. Some foods, such as beans and grains, need to be cooked, fermented or sprouted to reduce lectin content. Some lectins are beneficial, such as CLEC11A, which promotes bone growth, while others may be powerful toxins such as ricin. Lectins may be disabled by specific mono- and oligosaccharides, which bind to ingested lectins from grains, legumes, nightshade plants, and dairy; binding can prevent their attachment to the carbohydr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peter Hermann Stillmark
Peter Hermann Stillmark (22 July 1860, Penza, Russia – 23 June 1923, Pärnu, Estonia) was a Baltic-German microbiologist. In 1888 at the University in Dorpat, now Tartu in Estonia under Professor Rudolf Kobert's supervision, Peter Hermann Stillmark (1860–1923) completed his doctoral thesis ''Über Ricin, ein giftiges Ferment aus den Samen von ''Ricinus comm.'' L. und einigen anderen Euphorbiaceen'', which is a description of the isolation of ricin, a poisonous protein component from castor beans. That event is internationally recognised as the birth of a new branch of science called lectinology. Lectins are any of a group of proteins, derived from plants, that can bind to specific oligosaccharides on the surfaces of cells, causing the cells to clump together. They can be used to identify mutant cells in cell cultures and to determine blood groups, as they can cause the agglutination of red blood cells. Lectins are found in seeds of legumes and other tissues, in which they ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ribosome-inactivating Protein
A ribosome-inactivating protein (RIP) is a protein synthesis inhibitor that acts at the eukaryotic ribosome. This protein family describes a large family of such proteins that work by acting as rRNA N-glycosylase (EC 3.2.2.22). They inactivate 60S ribosomal subunits by an N-glycosidic cleavage, which releases a specific adenine base from the sugar-phosphate backbone of 28S rRNA. RIPs exist in bacteria and plants. Members of the family include shiga toxins, and type I (e.g. trichosanthin and luffin) and type II (e.g. ricin, agglutinin, and abrin) ribosome inactivating proteins (RIPs). All these toxins are structurally related. RIPs have been of considerable interest because of their potential use, conjugated with monoclonal antibodies, as immunotoxins to treat cancers. Further, trichosanthin has been shown to have potent activity against HIV-1-infected T cells and macrophages. Elucidation of the structure-function relationships of RIPs has therefore become a major research effo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dalton (unit)
The dalton or unified atomic mass unit (symbols: Da or u) is a non-SI unit of mass widely used in physics and chemistry. It is defined as of the mass of an unbound neutral atom of carbon-12 in its nuclear and electronic ground state and at rest. The atomic mass constant, denoted ''m''u, is defined identically, giving . This unit is commonly used in physics and chemistry to express the mass of atomic-scale objects, such as atoms, molecules, and elementary particles, both for discrete instances and multiple types of ensemble averages. For example, an atom of helium-4 has a mass of . This is an intrinsic property of the isotope and all helium-4 atoms have the same mass. Acetylsalicylic acid (aspirin), , has an average mass of approximately . However, there are no acetylsalicylic acid molecules with this mass. The two most common masses of individual acetylsalicylic acid molecules are , having the most common isotopes, and , in which one carbon is carbon-13. The molecular ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Quaternary Structure
Protein quaternary structure is the fourth (and highest) classification level of protein structure. Protein quaternary structure refers to the structure of proteins which are themselves composed of two or more smaller protein chains (also referred to as subunits). Protein quaternary structure describes the number and arrangement of multiple folded protein subunits in a multi-subunit complex. It includes organizations from simple dimers to large homooligomers and complexes with defined or variable numbers of subunits. In contrast to the first three levels of protein structure, not all proteins will have a quaternary structure since some proteins function as single units. Protein quaternary structure can also refer to biomolecular complexes of proteins with nucleic acids and other cofactors. Description and examples Many proteins are actually assemblies of multiple polypeptide chains. The quaternary structure refers to the number and arrangement of the protein subunits wit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycoside Hydrolase
Glycoside hydrolases (also called glycosidases or glycosyl hydrolases) catalyze the hydrolysis of glycosidic bonds in complex sugars. They are extremely common enzymes with roles in nature including degradation of biomass such as cellulose (cellulase), hemicellulose, and starch (amylase), in anti-bacterial defense strategies (e.g., lysozyme), in pathogenesis mechanisms (e.g., viral neuraminidases) and in normal cellular function (e.g., trimming mannosidases involved in N-linked glycoprotein biosynthesis). Together with glycosyltransferases, glycosidases form the major catalytic machinery for the synthesis and breakage of glycosidic bonds. Occurrence and importance Glycoside hydrolases are found in essentially all domains of life. In prokaryotes, they are found both as intracellular and extracellular enzymes that are largely involved in nutrient acquisition. One of the important occurrences of glycoside hydrolases in bacteria is the enzyme beta-galactosidase (LacZ), which ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Signal Peptide
A signal peptide (sometimes referred to as signal sequence, targeting signal, localization signal, localization sequence, transit peptide, leader sequence or leader peptide) is a short peptide (usually 16-30 amino acids long) present at the N-terminus (or occasionally nonclassically at the C-terminus or internally) of most newly synthesized proteins that are destined toward the secretory pathway. These proteins include those that reside either inside certain organelles (the endoplasmic reticulum, Golgi or endosomes), secreted from the cell, or inserted into most cellular membranes. Although most type I membrane-bound proteins have signal peptides, the majority of type II and multi-spanning membrane-bound proteins are targeted to the secretory pathway by their first transmembrane domain, which biochemically resembles a signal sequence except that it is not cleaved. They are a kind of target peptide. Function (translocation) Signal peptides function to prompt a cell to transloca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polypeptide
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides. A polypeptide is a longer, continuous, unbranched peptide chain. Hence, peptides fall under the broad chemical classes of biological polymers and oligomers, alongside nucleic acids, oligosaccharides, polysaccharides, and others. A polypeptide that contains more than approximately 50 amino acids is known as a protein. Proteins consist of one or more polypeptides arranged in a biologically functional way, often bound to ligands such as coenzymes and cofactors, or to another protein or other macromolecule such as DNA or RNA, or to complex macromolecular assemblies. Amino acids that have been incorporated into peptides are termed residues. A water molecule is released during formation of each amide bond.. All peptides except cy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Endopeptidase
Endopeptidase or endoproteinase are proteolytic peptidases that break peptide bonds of nonterminal amino acids (i.e. within the molecule), in contrast to exopeptidases, which break peptide bonds from end-pieces of terminal amino acids. For this reason, endopeptidases cannot break down peptides into monomers, while exopeptidases can break down proteins into monomers. A particular case of endopeptidase is the oligopeptidase, whose substrates are oligopeptides instead of proteins. They are usually very specific for certain amino acids. Examples of endopeptidases include: * Trypsin - cuts after Arg or Lys, unless followed by Pro. Very strict. Works best at pH 8. * Chymotrypsin - cuts after Phe, Trp, or Tyr, unless followed by Pro. Cuts more slowly after His, Met or Leu. Works best at pH 8. * Elastase - cuts after Ala, Gly, Ser, or Val, unless followed by Pro. * Thermolysin - cuts ''before'' Ile, Met, Phe, Trp, Tyr, or Val, unless ''preceded'' by Pro. Sometimes cuts after Ala, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Golgi Apparatus
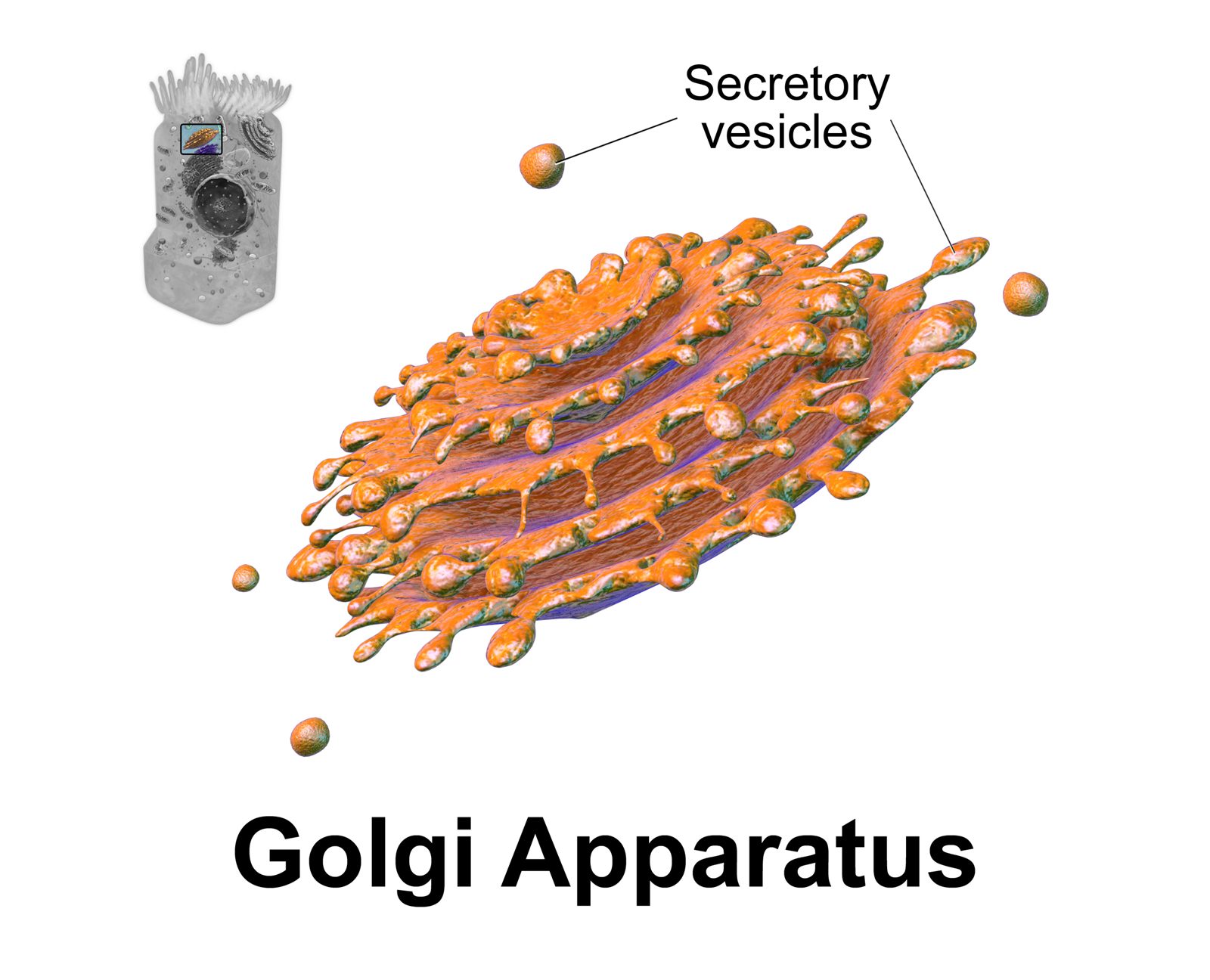
The Golgi apparatus (), also known as the Golgi complex, Golgi body, or simply the Golgi, is an organelle found in most eukaryotic cells. Part of the endomembrane system in the cytoplasm, it packages proteins into membrane-bound vesicles inside the cell before the vesicles are sent to their destination. It resides at the intersection of the secretory, lysosomal, and endocytic pathways. It is of particular importance in processing proteins for secretion, containing a set of glycosylation enzymes that attach various sugar monomers to proteins as the proteins move through the apparatus. It was identified in 1897 by the Italian scientist Camillo Golgi and was named after him in 1898. Discovery Owing to its large size and distinctive structure, the Golgi apparatus was one of the first organelles to be discovered and observed in detail. It was discovered in 1898 by Italian physician Camillo Golgi during an investigation of the nervous system. After first observing it under h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |