|
Renal Urea Handling
Renal urea handling is the part of renal physiology that deals with the reabsorption and secretion of urea. Movement of large amounts of urea across cell membranes is made possible by urea transporter proteins. Urea allows the kidneys to create hyperosmotic urine (urine that has more ions in it - is "more concentrated" - than that same person's blood plasma). Preventing the loss of water in this manner is important if the person's body must save water in order to maintain a suitable blood pressure or (more likely) in order to maintain a suitable concentration of sodium ions in the blood plasma. About 40% of the urea filtered is normally found in the final urine, since there is more reabsorption than secretion along the nephron. It is regulated by antidiuretic hormone, which controls the amount reabsorbed in the collecting duct system and secreted into the loop of Henle In the kidney, the loop of Henle () (or Henle's loop, Henle loop, nephron loop or its Latin counterpart ''an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Renal Physiology
Renal physiology (Latin ''rēnēs'', "kidneys") is the study of the physiology of the kidney. This encompasses all functions of the kidney, including maintenance of acid-base balance; regulation of fluid balance; regulation of sodium, potassium, and other electrolytes; clearance of toxins; absorption of glucose, amino acids, and other small molecules; regulation of blood pressure; production of various hormones, such as erythropoietin; and activation of vitamin D. Much of renal physiology is studied at the level of the nephron, the smallest functional unit of the kidney. Each nephron begins with a filtration component that filters the blood entering the kidney. This filtrate then flows along the length of the nephron, which is a tubular structure lined by a single layer of specialized cells and surrounded by capillaries. The major functions of these lining cells are the reabsorption of water and small molecules from the filtrate into the blood, and the secretion of wastes f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Descending Limb Of Loop Of Henle
Within the nephron of the kidney, the descending limb of loop of Henle is the portion of the renal tubule constituting the first part of the loop of Henle. Physiology The permeability is as follows: Also, the medullary interstitium is highly concentrated (because of the activity of the ascending limb), leading to a strong osmotic gradient from the descending limb to the medulla. Because of these factors, the concentration of the urine increases dramatically in the descending limb. Osmolality can reach up to 1400 mOsmol/kg by the end of the descending limb. Histology The epithelium of the Thick segment is low simple cuboidal epithelium. The epithelium of the Thin segment is simple squamous. They can be distinguished from the vasa recta by the absence of blood, and they can be distinguished from the thick ascending limb by the thickness of the epithelium. Nomenclature Like the ascending limb, the descending limb has thick and thin portions. However, this distinction is not ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Basolateral Membrane
Epithelial polarity is one example of the cell polarity that is a fundamental feature of many types of cells. Epithelial cells feature distinct 'apical', 'lateral' and 'basal' plasma membrane domains. Epithelial cells connect to one another via their lateral membranes to form epithelial sheets that line cavities and surfaces throughout the animal body. Each plasma membrane domain has a distinct protein composition, giving them distinct properties and allowing directional transport of molecules across the epithelial sheet. How epithelial cells generate and maintain polarity remains unclear, but certain molecules have been found to play a key role. A variety of molecules are located at the apical membrane, but only a few key molecules act as determinants that are required to maintain the identity of the apical membrane and, thus, epithelial polarity. These molecules are the proteins Cdc42, atypical protein kinase C (aPKC), Par6, Par3/Bazooka/ASIP. Crumbs, "Stardust" and protei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Urea Transporter 1
Urea transporter 1 is a protein that in humans is encoded by the ''SLC14A1'' gene. Function The SLC14A1 codes for a urea transporter (UTB) that is expressed in erythrocytes and kidney. SLC14A2 and SLC14A1 constitute solute carrier family 14. UTB proteins constitute the Kidd antigen system The Kidd antigen system (also known as Jk antigen) are proteins found in the Kidd's blood group, which act as antigens, i.e., they have the ability to produce antibodies under certain circumstances. The Jk antigen is found on a protein responsible .... References Further reading * * * * * * * * * * * * * * * * Solute carrier family {{membrane-protein-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Urea Transporter 2
Urea transporter 2 is a protein that in humans is encoded by the ''SLC14A2'' gene. Function In mammalian cells, urea is the chief end-product of nitrogen catabolism and plays an important role in the urinary concentration mechanism. Thus, the plasma membrane of erythrocytes and some renal epithelial cells exhibit an elevated urea permeability that is mediated by highly selective urea transporters. In mammals, two urea transporters have been identified: the renal tubular urea transporter, UT2 ( UT-A), and the erythrocyte urea transporter, UT11 (also called UT-B, coded for by the SLC14A1 gene). SLC14A2 and SLC14A1 Urea transporter 1 is a protein that in humans is encoded by the ''SLC14A1'' gene. Function The SLC14A1 codes for a urea transporter (UTB) that is expressed in erythrocytes and kidney. SLC14A2 and SLC14A1 constitute solute carrier family 14. UTB ... constitute solute carrier family 14. References Further reading * * * * * * * * * Solute carrier family [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solvent Drag
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for polar molecules and the most common solvent used by living things; all the ions and proteins in a cell are dissolved in water within the cell. The quantity of solute that can dissolve in a specific volume of solvent varies with temperature. Major uses of solvents are in paints, paint removers, inks, and dry cleaning. Specific uses for organic solvents are in dry cleaning (e.g. tetrachloroethylene); as paint thinners (toluene, turpentine); as nail polish removers and solvents of glue (acetone, methyl acetate, ethyl acetate); in spot removers (hexane, petrol ether); in detergents ( citrus terpenes); and in perfumes (ethanol). Solvents find various applications in chemical, pharmaceutical, oil, and gas industries, including in chemical synth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Apical Membrane
The cell membrane (also known as the plasma membrane (PM) or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of all cells from the outside environment (the extracellular space). The cell membrane consists of a lipid bilayer, made up of two layers of phospholipids with cholesterols (a lipid component) interspersed between them, maintaining appropriate membrane fluidity at various temperatures. The membrane also contains membrane proteins, including integral proteins that span the membrane and serve as membrane transporters, and peripheral proteins that loosely attach to the outer (peripheral) side of the cell membrane, acting as enzymes to facilitate interaction with the cell's environment. Glycolipids embedded in the outer lipid layer serve a similar purpose. The cell membrane controls the movement of substances in and out of cells and organelles, being selectively permeable to ions an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrochemical Potential
In electrochemistry, the electrochemical potential (ECP), ', is a thermodynamic measure of chemical potential that does not omit the energy contribution of electrostatics. Electrochemical potential is expressed in the unit of J/ mol. Introduction Each chemical species (for example, "water molecules", "sodium ions", "electrons", etc.) has an electrochemical potential (a quantity with units of energy) at any given point in space, which represents how easy or difficult it is to add more of that species to that location. If possible, a species will move from areas with higher electrochemical potential to areas with lower electrochemical potential; in equilibrium, the electrochemical potential will be constant everywhere for each species (it may have a different value for different species). For example, if a glass of water has sodium ions (Na+) dissolved uniformly in it, and an electric field is applied across the water, then the sodium ions will tend to get pulled by the electric fie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
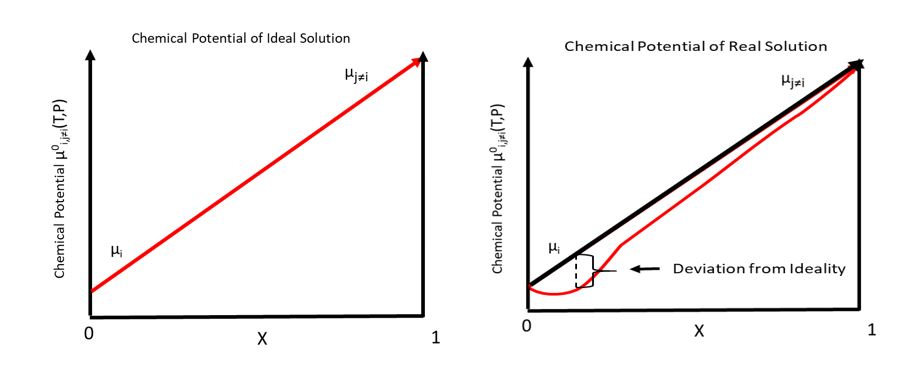
Chemical Potential
In thermodynamics, the chemical potential of a species is the energy that can be absorbed or released due to a change of the particle number of the given species, e.g. in a chemical reaction or phase transition. The chemical potential of a species in a mixture is defined as the rate of change of free energy of a thermodynamic system with respect to the change in the number of atoms or molecules of the species that are added to the system. Thus, it is the partial derivative of the free energy with respect to the amount of the species, all other species' concentrations in the mixture remaining constant. When both temperature and pressure are held constant, and the number of particles is expressed in moles, the chemical potential is the partial molar Gibbs free energy. At chemical equilibrium or in phase equilibrium, the total sum of the product of chemical potentials and stoichiometric coefficients is zero, as the free energy is at a minimum. In a system in diffusion equilibrium, th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Volt
The volt (symbol: V) is the unit of electric potential, electric potential difference (voltage), and electromotive force in the International System of Units (SI). It is named after the Italian physicist Alessandro Volta (1745–1827). Definition One volt is defined as the electric potential between two points of a conducting wire when an electric current of one ampere dissipates one watt of power between those points. Equivalently, it is the potential difference between two points that will impart one joule of energy per coulomb of charge that passes through it. It can be expressed in terms of SI base units ( m, kg, second, s, and ampere, A) as : \text = \frac = \frac = \frac. It can also be expressed as amperes times ohms (current times resistance, Ohm's law), webers per second (magnetic flux per time), watts per ampere (power per current), or joules per coulomb (energy per charge), which is also equivalent to electronvolts per elementary charge: : \text = \tex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Membrane Potential
Membrane potential (also transmembrane potential or membrane voltage) is the difference in electric potential between the interior and the exterior of a biological cell. That is, there is a difference in the energy required for electric charges to move from the internal to exterior cellular environments and vice versa, as long as there is no acquisition of kinetic energy or the production of radiation. The concentration gradients of the charges directly determine this energy requirement. For the exterior of the cell, typical values of membrane potential, normally given in units of milli volts and denoted as mV, range from –80 mV to –40 mV. All animal cells are surrounded by a membrane composed of a lipid bilayer with proteins embedded in it. The membrane serves as both an insulator and a diffusion barrier to the movement of ions. Transmembrane proteins, also known as ion transporter or ion pump proteins, actively push ions across the membrane and establish concentration gradi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mole (unit)
The mole, symbol mol, is the unit of amount of substance in the International System of Units (SI). The quantity amount of substance is a measure of how many elementary entities of a given substance are in an object or sample. The mole is defined as containing exactly elementary entities. Depending on what the substance is, an elementary entity may be an atom, a molecule, an ion, an ion pair, or a subatomic particle such as an electron. For example, 10 moles of water (a chemical compound) and 10 moles of mercury (a chemical element), contain equal amounts of substance and the mercury contains exactly one atom for each molecule of the water, despite the two having different volumes and different masses. The number of elementary entities in one mole is known as the Avogadro number, which is the approximate number of nucleons (protons or neutrons) in one gram of ordinary matter. The previous definition of a mole was simply the number of elementary entities equal to that of 12 gram ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |