|
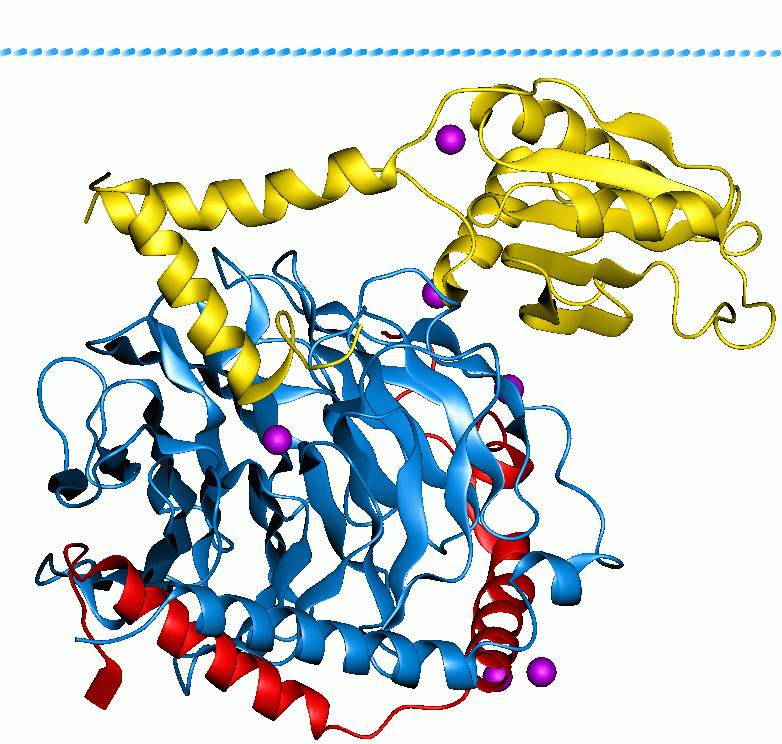
Retinylidene Protein
Retinylidene proteins, are proteins that use retinal as a chromophore for light reception. They are the molecular basis for a variety of light-sensing systems from phototaxis in flagellates to eyesight in animals. Retinylidene proteins include all forms of opsin and rhodopsin (in the broad sense). While rhodopsin in the narrow sense refers to a dim-light visual pigment found in vertebrates, usually on rod cells, ''rhodopsin'' in the broad sense (as used here) refers to any molecule consisting of an opsin and a retinal chromophore in the ground state. When activated by light, the chromophore is isomerized, at which point the molecule as a whole is no longer rhodopsin, but a related molecule such as metarhodopsin. However, it remains a retinylidene protein. The chromophore then separates from the opsin, at which point the bare opsin is a retinylidene protein. Thus, the molecule remains a retinylidene protein throughout the phototransduction cycle. Structure All rhodopsins consi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Retinal
Retinal (also known as retinaldehyde) is a polyene chromophore. Retinal, bound to proteins called opsins, is the chemical basis of visual phototransduction, the light-detection stage of visual perception (vision). Some microorganisms use retinal to convert light into metabolic energy. In fact, a recent study suggests most living organisms on our planet ~3 billion years ago used retinal to convert sunlight into energy rather than chlorophyll. Since retinal absorbs mostly green light and transmits purple light, this gave rise to the Purple Earth Hypothesis. There are many forms of vitamin A — all of which are converted to retinal, which cannot be made without them. Retinal itself is considered to be a form of vitamin A when eaten by an animal. The number of different molecules that can be converted to retinal varies from species to species. Retinal was originally called retinene, and was renamed after it was discovered to be vitamin A aldehyde. Vertebrate animals ingest reti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photoisomerization
In chemistry, photoisomerization is a form of isomerization induced by photoexcitation. Both reversible and irreversible photoisomerizations are known for photoswitchable compounds. The term "photoisomerization" usually, however, refers to a reversible process. Applications Photoisomerization of the compound retinal in the eye allows for vision. Photoisomerizable substrates have been put to practical use, for instance, in pigments for rewritable CDs, DVDs, and 3D optical data storage solutions. In addition, interest in photoisomerizable molecules has been aimed at molecular devices, such as molecular switches, molecular motors, and molecular electronics. Another class of device that uses the photoisomerization process is as an additive in liquid crystals to change their linear and nonlinear properties. Due to the photoisomerization is possible to induce a molecular reorientation in the liquid crystal bulk, which is used in holography, as spatial filter or optical switching. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Archaerhodopsin
Archaerhodopsin proteins are a family of retinal-containing photoreceptors found in the archaea genera '' Halobacterium'' and ''Halorubrum''. Like the homologous bacteriorhodopsin (bR) protein, archaerhodopsins harvest energy from sunlight to pump H+ ions out of the cell, establishing a proton motive force that is used for ATP synthesis. They have some structural similarities to the mammalian GPCR protein rhodopsin, but are not true homologs. Archaerhodopsins differ from bR in that the claret membrane, in which they are expressed, includes bacterioruberin, a second chromophore thought to protect against photobleaching. bR also lacks the omega loop structure that has been observed at the N-terminus of the structures of several archaerhodopsins. Mutants of Archaerhodopsin-3 (AR3) are widely used as tools in optogenetics for neuroscience research. Etymology The term ''archaerhodopsin'' is a portmanteau of archaea (the domain in which the proteins are found) and rhodopsin (a ph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteorhodopsin
Proteorhodopsin (also known as pRhodopsin) is a family of transmembrane proteins that use retinal as a chromophore for light-mediated functionality, in this case, a proton pump. pRhodopsin is found in marine planktonic bacteria, archaea and eukaryotes ( protae), but was first discovered in bacteria. Its name is derived from proteobacteria (now called Pseudomonadota) that were named after Ancient Greek Πρωτεύς (Proteus), an early sea god mentioned by Homer as "Old Man of the Sea", Ῥόδος (rhódon) for "rose", due to its pinkish color, and ὄψις (opsis) for "sight". Some members of the family, Homologous rhodopsin-like pigments, i.e. bacteriorhodopsin (of which there are more than 800 types) have Sensory Functions like opsins, integral for visual phototransduction. Many of these sensory functions are unknown – for example, the function of Neuropsin in the human retina. Members are known to have different absorption spectra including green and blue vis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Halorhodopsin
Halorhodopsin is a light-gated ion pump, specific for chloride ions, found in archaea, known as halobacteria. It is a seven-transmembrane retinylidene protein from microbial rhodopsin family. It is similar in tertiary structure (but not primary sequence structure) to vertebrate rhodopsins, the pigments that sense light in the retina. Halorhodopsin also shares sequence similarity to channelrhodopsin, another light-driven ion channel. Halorhodopsin contains the essential light-isomerizable vitamin A derivative all-trans-retinal. Due to the intense attention on solving the structure and function of this molecule, halorhodopsin is one of the few membrane proteins whose crystal structure is known. Halorhodopsin uses the energy of green/yellow light to move chloride ions into the cell, overcoming the membrane potential. Beside chlorides it transports other halides and nitrates into the cell. Potassium chloride uptake by cells helps to maintain osmotic balance during cell growth. By ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bacteriorhodopsin
Bacteriorhodopsin is a protein used by Archaea, most notably by haloarchaea, a class of the Euryarchaeota. It acts as a proton pump; that is, it captures light energy and uses it to move protons across the membrane out of the cell. The resulting proton gradient is subsequently converted into chemical energy. Function Bacteriorhodopsin is a light-driven H+ ion transporter found in some haloarchaea, most notably '' Halobacterium salinarum'' (formerly known as syn. ''H. halobium''). The proton-motive force generated by the protein is used by ATP synthase to generate adenosine triphosphate (ATP). By expressing bacteriorhodopsin, the archaea cells are able to synthesise ATP in the absence of a carbon source. Structure Bacteriorhodopsin is a 27 kDa integral membrane protein usually found in two-dimensional crystalline patches known as "purple membrane", which can occupy almost 50% of the surface area of the archaeal cell. The repeating element of the hexagonal lattice is compos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Channelrhodopsin
Channelrhodopsins are a subfamily of retinylidene proteins ( rhodopsins) that function as light-gated ion channels. They serve as sensory photoreceptors in unicellular green algae, controlling phototaxis: movement in response to light. Expressed in cells of other organisms, they enable light to control electrical excitability, intracellular acidity, calcium influx, and other cellular processes (see optogenetics). Channelrhodopsin-1 (ChR1) and Channelrhodopsin-2 (ChR2) from the model organism ''Chlamydomonas reinhardtii'' are the first discovered channelrhodopsins. Variants that are sensitive to different colors of light or selective for specific ions (ACRs, KCRs) have been cloned from other species of algae and protists. History Phototaxis and photoorientation of microalgae have been studied over more than hundred years in many laboratories worldwide. In 1980, Ken Foster developed the first consistent theory about the functionality of algal eyes. He also analyzed published ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transmembrane Helix
A transmembrane domain (TMD) is a membrane-spanning protein domain. TMDs generally adopt an alpha helix topological conformation, although some TMDs such as those in porins can adopt a different conformation. Because the interior of the lipid bilayer is hydrophobic, the amino acid residues in TMDs are often hydrophobic, although proteins such as membrane pumps and ion channels can contain polar residues. TMDs vary greatly in length, sequence, and hydrophobicity, adopting organelle-specific properties. Functions of transmembrane domains Transmembrane domains are known to perform a variety of functions. These include: * Anchoring transmembrane proteins to the membrane. *Facilitating molecular transport of molecules such as ions and proteins across biological membranes; usually hydrophilic residues and binding sites in the TMDs help in this process. * Signal transduction across the membrane; many transmembrane proteins, such as G protein-coupled receptors, receive extracellul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
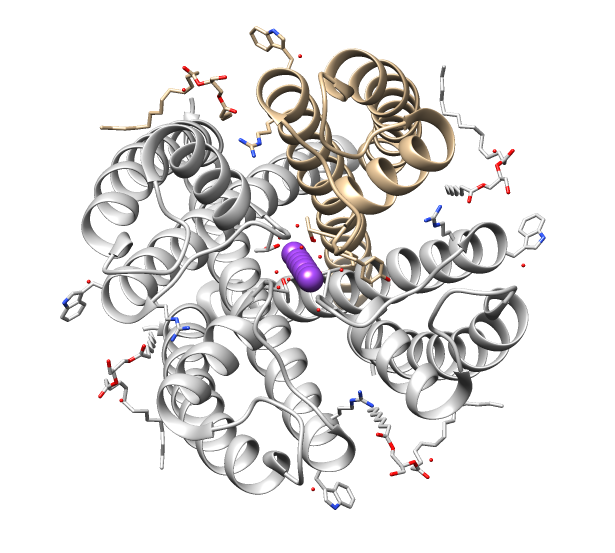
Microbial Rhodopsin
Microbial rhodopsins, also known as bacterial rhodopsins are retinal-binding proteins that provide light-dependent ion transport and sensory functions in halophilic and other bacteria. They are integral membrane proteins with seven transmembrane helices, the last of which contains the attachment point (a conserved lysine) for retinal. This protein family includes light-driven proton pumps, ion pumps and ion channels, as well as light sensors. For example, the proteins from halobacteria include bacteriorhodopsin and archaerhodopsin, which are light-driven proton pumps; halorhodopsin, a light-driven chloride pump; and sensory rhodopsin, which mediates both photoattractant (in the red) and photophobic (in the ultra-violet) responses. Proteins from other bacteria include proteorhodopsin. Contrary to their name, microbial rhodopsins are found not only in Archaea and Bacteria, but also in Eukaryota (such as algae) and viruses; although they are rare in complex multicellular org ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
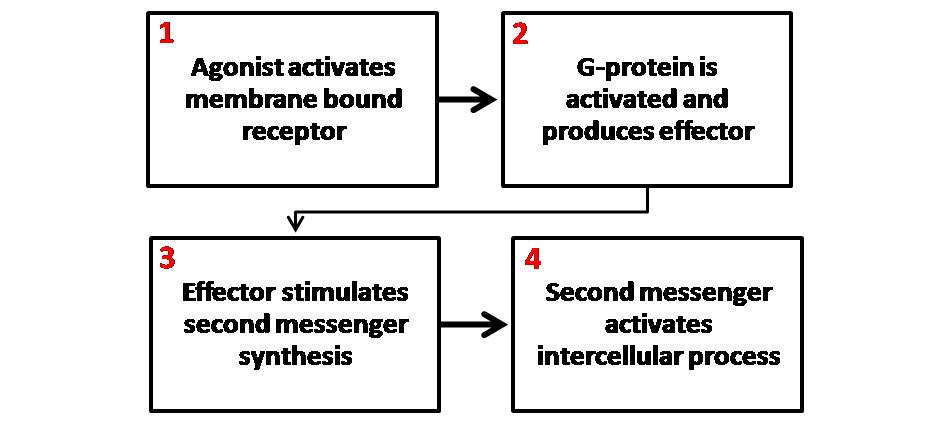
Second Messenger
Second messengers are intracellular signaling molecules released by the cell in response to exposure to extracellular signaling molecules—the first messengers. (Intercellular signals, a non-local form or cell signaling, encompassing both first messengers and second messengers, are classified as autocrine, juxtacrine, paracrine, and endocrine depending on the range of the signal.) Second messengers trigger physiological changes at cellular level such as proliferation, differentiation, migration, survival, apoptosis and depolarization. They are one of the triggers of intracellular signal transduction cascades. Examples of second messenger molecules include cyclic AMP, cyclic GMP, inositol triphosphate, diacylglycerol, and calcium. First messengers are extracellular factors, often hormones or neurotransmitters, such as epinephrine, growth hormone, and serotonin. Because peptide hormones and neurotransmitters typically are biochemically hydrophilic molecules, these first mess ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
G Protein
G proteins, also known as guanine nucleotide-binding proteins, are a family of proteins that act as molecular switches inside cells, and are involved in transmitting signals from a variety of stimuli outside a cell to its interior. Their activity is regulated by factors that control their ability to bind to and hydrolyze guanosine triphosphate (GTP) to guanosine diphosphate (GDP). When they are bound to GTP, they are 'on', and, when they are bound to GDP, they are 'off'. G proteins belong to the larger group of enzymes called GTPases. There are two classes of G proteins. The first function as monomeric small GTPases (small G-proteins), while the second function as heterotrimeric G protein complexes. The latter class of complexes is made up of '' alpha'' (α), ''beta'' (β) and ''gamma'' (γ) subunits. In addition, the beta and gamma subunits can form a stable dimeric complex referred to as the beta-gamma complex . Heterotrimeric G proteins located within the cell are activ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ion Channel
Ion channels are pore-forming membrane proteins that allow ions to pass through the channel pore. Their functions include establishing a resting membrane potential, shaping action potentials and other electrical signals by gating the flow of ions across the cell membrane, controlling the flow of ions across secretory and epithelial cells, and regulating cell volume. Ion channels are present in the membranes of all cells. Ion channels are one of the two classes of ionophoric proteins, the other being ion transporters. The study of ion channels often involves biophysics, electrophysiology, and pharmacology, while using techniques including voltage clamp, patch clamp, immunohistochemistry, X-ray crystallography, fluoroscopy, and RT-PCR. Their classification as molecules is referred to as channelomics. Basic features There are two distinctive features of ion channels that differentiate them from other types of ion transporter proteins: #The rate of ion transport through the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |






.png)
.jpg)