|
Radiogenic Nuclide
A radiogenic nuclide is a nuclide that is produced by a process of radioactive decay. It may itself be radioactive (a radionuclide) or stable (a stable nuclide). Radiogenic nuclides (more commonly referred to as radiogenic isotopes) form some of the most important tools in geology. They are used in two principal ways: #In comparison with the quantity of the radioactive 'parent isotope' in a system, the quantity of the radiogenic 'daughter product' is used as a radiometric dating tool (e.g. uranium–lead geochronology). #In comparison with the quantity of a non-radiogenic isotope of the same element, the quantity of the radiogenic isotope is used to define its isotopic signature (e.g. 206Pb/204Pb). This technique is discussed in more detail under the heading isotope geochemistry. Examples Some naturally occurring isotopes are entirely radiogenic, but all these are isotopes that are radioactive, with half-lives too short to occur primordially. Thus, they are only present as ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclide
A nuclide (or nucleide, from nucleus, also known as nuclear species) is a class of atoms characterized by their number of protons, ''Z'', their number of neutrons, ''N'', and their nuclear energy state. The word ''nuclide'' was coined by Truman P. Kohman in 1947. Kohman defined ''nuclide'' as a "species of atom characterized by the constitution of its nucleus" containing a certain number of neutrons and protons. The term thus originally focused on the nucleus. Nuclides vs isotopes A nuclide is a species of an atom with a specific number of protons and neutrons in the nucleus, for example carbon-13 with 6 protons and 7 neutrons. The nuclide concept (referring to individual nuclear species) emphasizes nuclear properties over chemical properties, while the isotope concept (grouping all atoms of each element) emphasizes chemical over nuclear. The neutron number has large effects on nuclear properties, but its effect on chemical reactions is negligible for most elements. Even in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon-14
Carbon-14, C-14, or radiocarbon, is a radioactive isotope of carbon with an atomic nucleus containing 6 protons and 8 neutrons. Its presence in organic materials is the basis of the radiocarbon dating method pioneered by Willard Libby and colleagues (1949) to date archaeological, geological and hydrogeological samples. Carbon-14 was discovered on February 27, 1940, by Martin Kamen and Sam Ruben at the University of California Radiation Laboratory in Berkeley, California. Its existence had been suggested by Franz N. D. Kurie, Franz Kurie in 1934. There are three naturally occurring isotopes of carbon on Earth: carbon-12 (), which makes up 99% of all carbon on Earth; carbon-13 (), which makes up 1%; and carbon-14 (), which occurs in trace amounts, making up about 1 or 1.5 atoms per 1012 atoms of carbon in the atmosphere. Carbon-12 and carbon-13 are both stable, while carbon-14 is unstable and has a half-life of 5,730 ± 40 years. Carbon-14 decays into nitrogen-14 () through bet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mantle (geology)
A mantle is a layer inside a planetary body bounded below by a Planetary core, core and above by a Crust (geology), crust. Mantles are made of Rock (geology), rock or Volatiles, ices, and are generally the largest and most massive layer of the planetary body. Mantles are characteristic of planetary bodies that have undergone planetary differentiation, differentiation by density. All Terrestrial planet, terrestrial planets (including Earth), a number of Asteroid, asteroids, and some planetary Natural satellite, moons have mantles. Earth's mantle The Earth's mantle is a layer of Silicate minerals, silicate rock between the Crust (geology), crust and the Earth's outer core, outer core. Its mass of 4.01 × 1024 kg is 67% the mass of the Earth. It has a thickness of making up about 84% of Earth's volume. It is predominantly solid, but in Geologic time scale, geological time it behaves as a Viscosity, viscous fluid. Partial melting of the mantle at mid-ocean ridges produ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
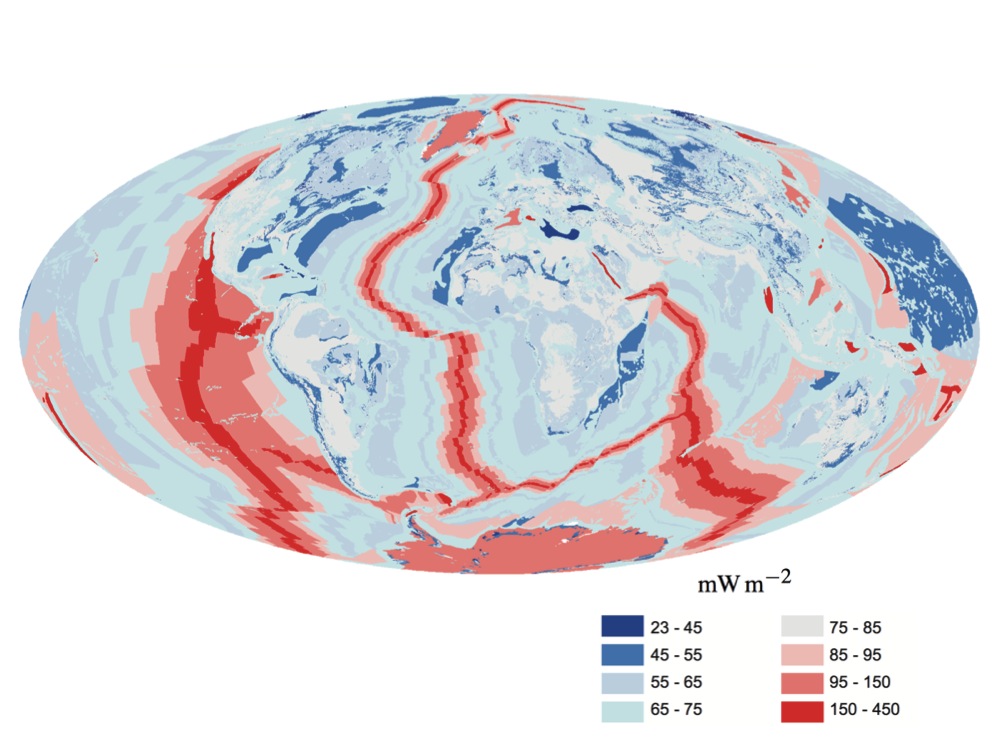
Earth's Internal Heat Budget
Earth's internal heat budget is fundamental to the thermal history of the Earth. The flow of heat from Earth's interior to the surface is estimated at 47±2 terawatts (TW)Davies, J. H., & Davies, D. R. (2010). Earth's surface heat flux. Solid Earth, 1(1), 5–24. and comes from two main sources in roughly equal amounts: the ''radiogenic heat'' produced by the radioactive decay of isotopes in the mantle and crust, and the ''primordial heat'' left over from the formation of Earth. Earth's internal heat travels along geothermal gradients and powers most geological processes. It drives mantle convection, plate tectonics, mountain building, rock metamorphism, and volcanism. Convective heat transfer within the planet's high-temperature metallic core is also theorized to sustain a geodynamo which generates Earth's magnetic field. Despite its geological significance, Earth's interior heat contributes only 0.03% of Earth's total energy budget at the surface, which is dominated by 1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable atoms survive. The term is also used more generally to characterize any type of exponential (or, rarely, non-exponential) decay. For example, the medical sciences refer to the biological half-life of drugs and other chemicals in the human body. The converse of half-life (in exponential growth) is doubling time. The original term, ''half-life period'', dating to Ernest Rutherford's discovery of the principle in 1907, was shortened to ''half-life'' in the early 1950s. Rutherford applied the principle of a radioactive element's half-life in studies of age determination of rocks by measuring the decay period of radium to lead-206. Half-life is constant over the lifetime of an exponentially decaying quantity, and it is a characteristic unit for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium-26
Aluminium-26 (26Al, Al-26) is a Radionuclide, radioactive isotope of the chemical element aluminium, decaying by either positron emission or electron capture to stable magnesium-26. The half-life of 26Al is 7.17 (717,000) years. This is far too short for the isotope to survive as a primordial nuclide, but a small amount of it is produced by collisions of atoms with cosmic ray protons. Decay of aluminium-26 also produces gamma rays and x-rays. The x-rays and Auger effect, Auger electrons are emitted by the excited atomic shell of the daughter 26Mg after the electron capture which typically leaves a hole in one of the lower sub-shells. Because it is radioactive, it is typically stored behind at least of lead. Contact with 26Al may result in radiological contamination necessitating special tools for transfer, use, and storage. Dating Aluminium-26 can be used to calculate the terrestrial age of meteorites and comets. It is produced in significant quantities in extraterrestrial obje ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodine-129
Iodine-129 (129I) is a long-lived radioisotope of iodine which occurs naturally, but also is of special interest in the monitoring and effects of man-made nuclear fission products, where it serves as both tracer and potential radiological contaminant. Formation and decay 129I is one of seven long-lived fission products. It is primarily formed from the fission of uranium and plutonium in nuclear reactors. Significant amounts were released into the atmosphere as a result of nuclear weapons testing in the 1950s and 1960s. It is also naturally produced in small quantities, due to the spontaneous fission of natural uranium, by cosmic ray spallation of trace levels of xenon in the atmosphere, and by cosmic ray muons striking tellurium-130. 129I decays with a half-life of 15.7 million years, with low-energy beta and gamma emissions, to stable xenon-129 (129Xe). Fission product 129I is one of the seven long-lived fission products that are produced in significant amounts. Its yield ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Extinct Radionuclide
An extinct radionuclide is a radionuclide that was formed by nucleosynthesis before the formation of the Solar System, about 4.6 billion years ago, but has since decayed to virtually zero abundance and is no longer detectable as a primordial nuclide. Extinct radionuclides were generated by various processes in the early Solar system, and became part of the composition of meteorites and protoplanets. All widely documented extinct radionuclides have half-lives shorter than 100 million years. Short-lived radioisotopes that are found in nature are continuously generated or replenished by natural processes, such as cosmic rays (cosmogenic nuclides), background radiation, or the decay chain or spontaneous fission of other radionuclides. Short-lived isotopes that are not generated or replenished by natural processes are not found in nature, so they are known as extinct radionuclides. Their former existence is inferred from a superabundance of their stable or nearly stable decay products ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Supernova
A supernova is a powerful and luminous explosion of a star. It has the plural form supernovae or supernovas, and is abbreviated SN or SNe. This transient astronomical event occurs during the last evolutionary stages of a massive star or when a white dwarf is triggered into runaway nuclear fusion. The original object, called the ''progenitor'', either collapses to a neutron star or black hole, or is completely destroyed. The peak optical luminosity of a supernova can be comparable to that of an entire galaxy before fading over several weeks or months. Supernovae are more energetic than novae. In Latin language, Latin, ''nova'' means "new", referring astronomically to what appears to be a temporary new bright star. Adding the prefix "super-" distinguishes supernovae from ordinary novae, which are far less luminous. The word ''supernova'' was coined by Walter Baade and Fritz Zwicky in 1929. The last supernova to be directly observed in the Milky Way was Kepler's Supernova in 160 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ternary Fission
Ternary fission is a comparatively rare (0.2 to 0.4% of events) type of nuclear fission in which three charged products are produced rather than two. As in other nuclear fission processes, other uncharged particles such as multiple neutrons and gamma rays are produced in ternary fission. Ternary fission may happen during neutron-induced fission or in spontaneous fission (the type of radioactive decay). About 25% more ternary fission happens in spontaneous fission compared to the same fission system formed after thermal neutron capture, illustrating that these processes remain physically slightly different, even after the absorption of the neutron, possibly because of the extra energy present in the nuclear reaction system of thermal neutron-induced fission. Quaternary fission, at 1 per 1000 million fissions, is also known (see below). Products The most common nuclear fission process is "binary fission." It produces two charged asymmetrical fission products with maximally pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tritium
Tritium ( or , ) or hydrogen-3 (symbol T or H) is a rare and radioactive isotope of hydrogen with half-life about 12 years. The nucleus of tritium (t, sometimes called a ''triton'') contains one proton and two neutrons, whereas the nucleus of the common isotope hydrogen-1 (''protium'') contains one proton and zero neutrons, and that of hydrogen-2 (''deuterium'') contains one proton and one neutron. Naturally occurring tritium is extremely rare on Earth. The atmosphere has only trace amounts, formed by the interaction of its gases with cosmic rays. It can be produced artificially by irradiating lithium metal or lithium-bearing ceramic pebbles in a nuclear reactor and is a low-abundance byproduct in normal operations of nuclear reactors. Tritium is used as the energy source in radioluminescent lights for watches, gun sights, numerous instruments and tools, and even novelty items such as self-illuminating key chains. It is used in a medical and scientific setting as a radioacti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helium-4
Helium-4 () is a stable isotope of the element helium. It is by far the more abundant of the two naturally occurring isotopes of helium, making up about 99.99986% of the helium on Earth. Its nucleus is identical to an alpha particle, and consists of two protons and two neutrons. Alpha decay of heavy elements in the Earth's crust is the source of most naturally occurring helium-4 on Earth, produced after the planet cooled and solidified. While it is also produced by nuclear fusion in stars, most helium-4 in the Sun and in the universe is thought to have been produced by the Big Bang, and is referred to as " primordial helium". However, primordial helium-4 is largely absent from the Earth, having escaped during the high-temperature phase of Earth's formation. Helium-4 makes up about one quarter of the ordinary matter in the universe by mass, with almost all of the rest being hydrogen. When liquid helium-4 is cooled to below , it becomes a superfluid, with properties that are ver ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |