|
Radiation Weighting Factor
Equivalent dose is a Dose (radiation), dose quantity '' H '' representing the stochastic health effects of low levels of ionizing radiation on the human body which represents the probability of radiation-induced cancer and genetic damage. It is derived from the physical quantity absorbed dose, but also takes into account the biological effectiveness of the radiation, which is dependent on the radiation type and energy. In the SI system of units, the unit of measure is the sievert (Sv). Application To enable consideration of stochastic health risk, calculations are performed to convert the physical quantity absorbed dose into equivalent dose, the details of which depend on the radiation type. For applications in radiation protection and dosimetry assessment, the International Commission on Radiological Protection (ICRP) and the International Commission on Radiation Units and Measurements (ICRU) have published recommendations and data on how to calculate equivalent dose from absorbe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sievert
The sievert (symbol: SvNot be confused with the sverdrup or the svedberg, two non-SI units that sometimes use the same symbol.) is a unit in the International System of Units (SI) intended to represent the stochastic health risk of ionizing radiation, which is defined as the probability of causing radiation-induced cancer and genetic damage. The sievert is important in dosimetry and radiation protection. It is named after Rolf Maximilian Sievert, a Swedish medical physicist renowned for work on radiation dose measurement and research into the biological effects of radiation. The sievert is used for radiation dose quantities such as equivalent dose and effective dose, which represent the risk of external radiation from sources outside the body, and committed dose, which represents the risk of internal irradiation due to inhaled or ingested radioactive substances. According to the International Commission on Radiological Protection (ICRP) one sievert results in a 5.5% probabilit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
X-ray
X-rays (or rarely, ''X-radiation'') are a form of high-energy electromagnetic radiation. In many languages, it is referred to as Röntgen radiation, after the German scientist Wilhelm Conrad Röntgen, who discovered it in 1895 and named it ''X-radiation'' to signify an unknown type of radiation.Novelline, Robert (1997). ''Squire's Fundamentals of Radiology''. Harvard University Press. 5th edition. . X-ray wavelengths are shorter than those of ultraviolet rays and longer than those of gamma rays. There is no universally accepted, strict definition of the bounds of the X-ray band. Roughly, X-rays have a wavelength ranging from 10 nanometers to 10 picometers, corresponding to frequencies in the range of 30 petahertz to 30 exahertz ( to ) and photon energies in the range of 100 eV to 100 keV, respectively. X-rays can penetrate many solid substances such as construction materials and living tissue, so X-ray radiography is widely used in medi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SI Unit
The International System of Units, known by the international abbreviation SI in all languages and sometimes Pleonasm#Acronyms and initialisms, pleonastically as the SI system, is the modern form of the metric system and the world's most widely used system of measurement. Established and maintained by the General Conference on Weights and Measures (CGPM), it is the only system of measurement with an official status in nearly every country in the world, employed in science, technology, industry, and everyday commerce. The SI comprises a Coherence (units of measurement), coherent system of units of measurement starting with seven SI base unit, base units, which are the second (symbol s, the unit of time), metre (m, length), kilogram (kg, mass), ampere (A, electric current), kelvin (K, thermodynamic temperature), Mole (unit), mole (mol, amount of substance), and candela (cd, luminous intensity). The system can accommodate coherent units for an unlimited number of additional qua ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Regulatory Commission
The Nuclear Regulatory Commission (NRC) is an independent agency of the United States government tasked with protecting public health and safety related to nuclear energy. Established by the Energy Reorganization Act of 1974, the NRC began operations on January 19, 1975, as one of two successor agencies to the United States Atomic Energy Commission. Its functions include overseeing reactor safety and security, administering reactor licensing and renewal, licensing radioactive materials, radionuclide safety, and managing the storage, security, recycling, and disposal of spent fuel. History Prior to 1975 the Atomic Energy Commission was in charge of matters regarding radionuclides. The AEC was dissolved, because it was perceived as unduly favoring the industry it was charged with regulating.John Byrne and Steven M. Hoffman (1996). ''Governing the Atom: The Politics of Risk'', Transaction Publishers, p. 163. The NRC was formed as an independent commission to oversee nuclear en ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
International Committee For Weights And Measures
The General Conference on Weights and Measures (GCWM; french: Conférence générale des poids et mesures, CGPM) is the supreme authority of the International Bureau of Weights and Measures (BIPM), the intergovernmental organization established in 1875 under the terms of the Metre Convention through which member states act together on matters related to measurement science and measurement standards. The CGPM is made up of delegates of the governments of the member states and observers from the Associates of the CGPM. Under its authority, the International Committee for Weights and Measures (ICWM; ) executes an exclusive direction and supervision of the BIPM. Initially the Metre Convention was only concerned with the kilogram and the metre, but in 1921 the scope of the treaty was extended to accommodate all physical measurements and hence all aspects of the metric system. In 1960 the 11th CGPM approved the International System of Units, usually known as "SI". The General ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ICRP
The International Commission on Radiological Protection (ICRP) is an independent, international, non-governmental organization, with the mission to protect people, animals, and the environment from the harmful effects of ionising radiation. Its recommendations form the basis of radiological protection policy, regulations, guidelines and practice worldwide. The ICRP was effectively founded in 1928 at the second International Congress of Radiology in Stockholm, Sweden but was then called the International X-ray and Radium Protection Committee (IXRPC). In 1950 it was restructured to take account of new uses of radiation outside the medical area and re-named as the ICRP. The ICRP is a sister organisation to the International Commission on Radiation Units and Measurements (ICRU). In general terms ICRU defines the units, and ICRP recommends, develops and maintains the International system of radiological protection which uses these units. Operation The ICRP is a not-for-profit org ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Nucleus
The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden experiments, Geiger–Marsden gold foil experiment. After the discovery of the neutron in 1932, models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. An atom is composed of a positively charged nucleus, with a cloud of negatively charged electrons surrounding it, bound together by electrostatic force. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud. Protons and neutrons are bound together to form a nucleus by the nuclear force. The diameter of the nucleus is in the range of () for hydrogen (the diameter of a single proton) to about for uranium. These dimensions are much smaller than the diameter of the atom itself (nucleus + electron cloud), by a factor of about 2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Fission Product
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the release of heat energy (kinetic energy of the nuclei), and gamma rays. The two smaller nuclei are the ''fission products''. (See also Fission products (by element)). About 0.2% to 0.4% of fissions are ternary fissions, producing a third light nucleus such as helium-4 (90%) or tritium (7%). The fission products themselves are usually unstable and therefore radioactive. Due to being relatively neutron-rich for their atomic number, many of them quickly undergo beta decay. This releases additional energy in the form of beta particles, antineutrinos, and gamma rays. Thus, fission events normally result in beta and gamma radiation, even though this radiation is not produced directly by the fission event itself. The produced radionuclides have va ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha Particle
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay, but may also be produced in other ways. Alpha particles are named after the first letter in the Greek alphabet, α. The symbol for the alpha particle is α or α2+. Because they are identical to helium nuclei, they are also sometimes written as or indicating a helium ion with a +2 charge (missing its two electrons). Once the ion gains electrons from its environment, the alpha particle becomes a normal (electrically neutral) helium atom . Alpha particles have a net spin of zero. Due to the mechanism of their production in standard alpha radioactive decay, alpha particles generally have a kinetic energy of about 5 MeV, and a velocity in the vicinity of 4% of the speed of light. (See discussion below for the limits of these figures in alpha decay.) T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pion
In particle physics, a pion (or a pi meson, denoted with the Greek letter pi: ) is any of three subatomic particles: , , and . Each pion consists of a quark and an antiquark and is therefore a meson. Pions are the lightest mesons and, more generally, the lightest hadrons. They are unstable, with the charged pions and decaying after a mean lifetime of 26.033 nanoseconds ( seconds), and the neutral pion decaying after a much shorter lifetime of 85 attoseconds ( seconds). Charged pions most often decay into muons and muon neutrinos, while neutral pions generally decay into gamma rays. The exchange of virtual pions, along with vector, rho and omega mesons, provides an explanation for the residual strong force between nucleons. Pions are not produced in radioactive decay, but commonly are in high-energy collisions between hadrons. Pions also result from some matter–antimatter annihilation events. All types of pions are also produced in natural pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
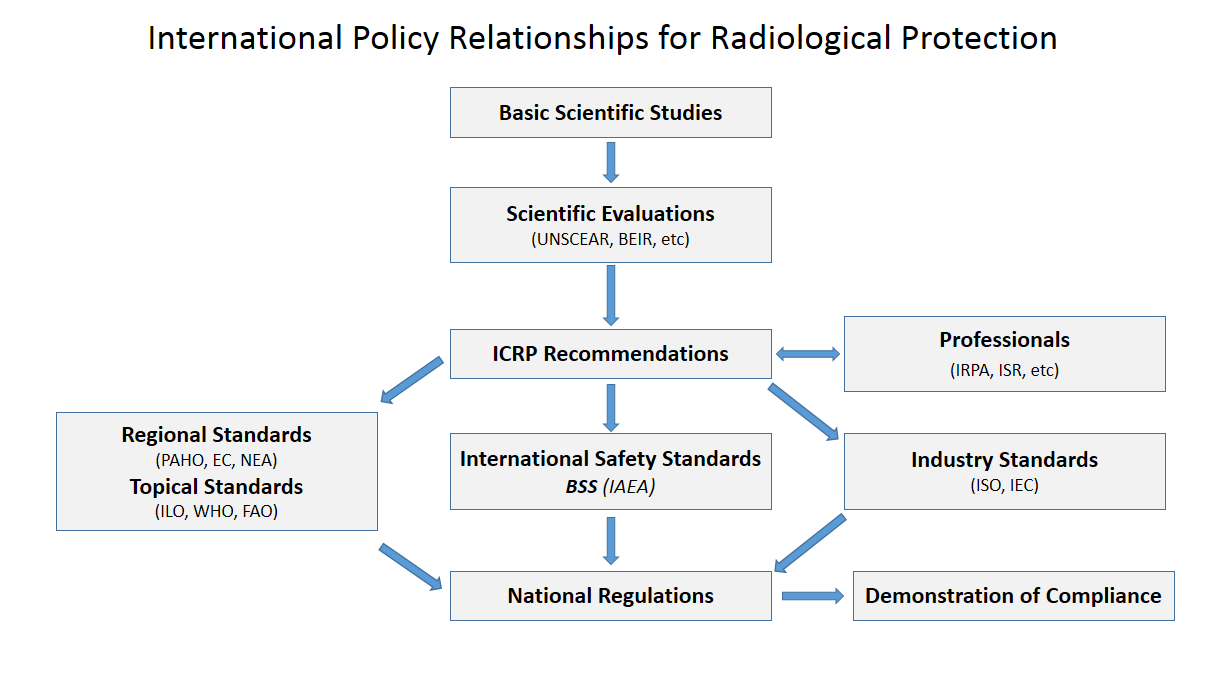
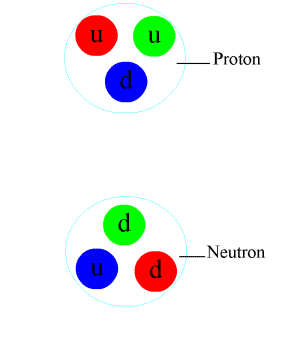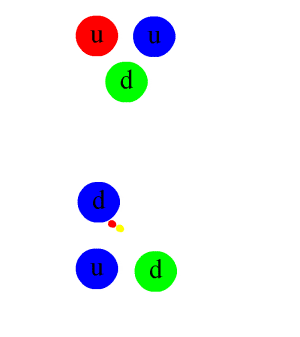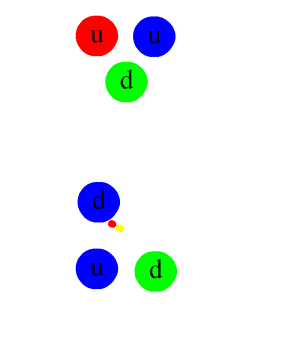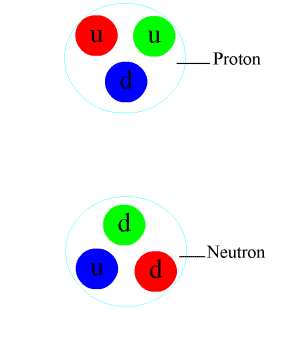
Proton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ratio). Protons and neutrons, each with masses of approximately one atomic mass unit, are jointly referred to as "nucleons" (particles present in atomic nuclei). One or more protons are present in the Atomic nucleus, nucleus of every atom. They provide the attractive electrostatic central force which binds the atomic electrons. The number of protons in the nucleus is the defining property of an element, and is referred to as the atomic number (represented by the symbol ''Z''). Since each chemical element, element has a unique number of protons, each element has its own unique atomic number, which determines the number of atomic electrons and consequently the chemical characteristics of the element. The word ''proton'' is Greek language, G ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons behave similarly within the nucleus, and each has a mass of approximately one atomic mass unit, they are both referred to as nucleons. Their properties and interactions are described by nuclear physics. Protons and neutrons are not elementary particles; each is composed of three quarks. The chemical properties of an atom are mostly determined by the configuration of electrons that orbit the atom's heavy nucleus. The electron configuration is determined by the charge of the nucleus, which is determined by the number of protons, or atomic number. The number of neutrons is the neutron number. Neutrons do not affect the electron configuration, but the sum of atomic and neutron numbers is the mass of the nucleus. Atoms of a chemical element t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



.jpg)