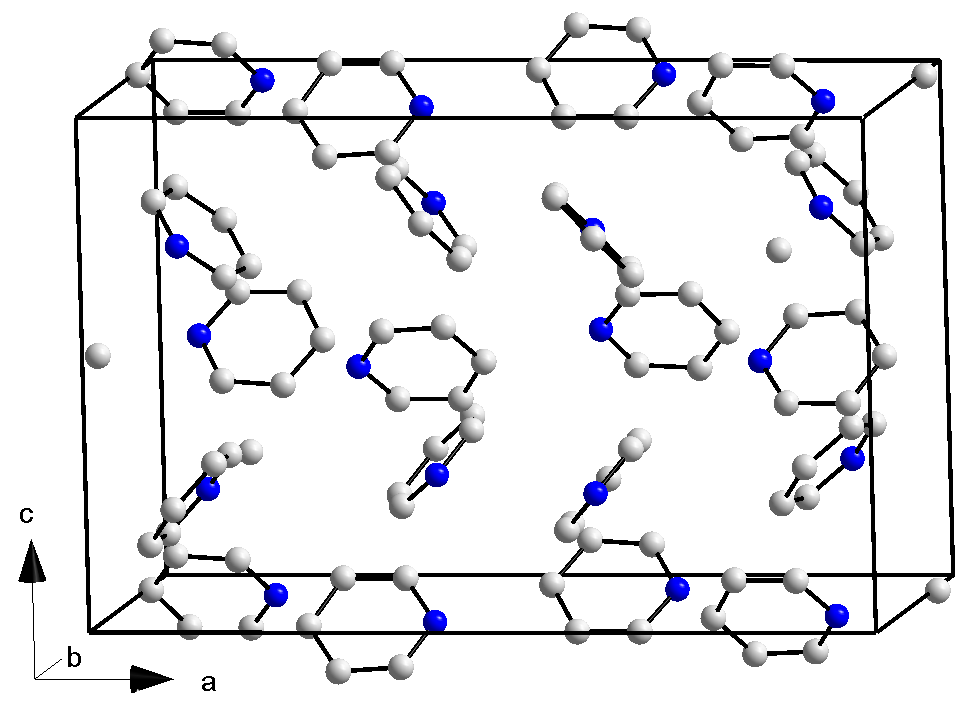
|
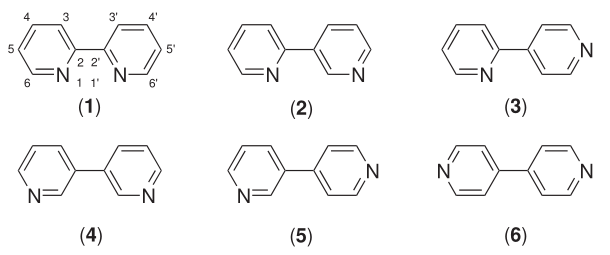
Quaterpyridine
Quaterpyridine refers to a family of pyridine derivatives with the formula (NC5H4-C5H3N)2. These compounds can also be viewed as bipyridine decorated with two pyridyl substituents. Several isomers are known. All are colorless solids. One particular isomer, 2,2':6',2'':6'',2-quaterpyridine, a derivative of 2,2'-bipyridine has attracted interest because it is a potential tetradentate ligand in coordination chemistry.{{cite journal , doi=10.1021/acs.chemrev.6b00377, title=Quaterpyridines as Scaffolds for Functional Metallosupramolecular Materials , year=2016 , last1=Gorczyński , first1=Adam , last2=Harrowfield , first2=Jack M. , last3=Patroniak , first3=Violetta , last4=Stefankiewicz , first4=Artur R. , journal=Chemical Reviews , volume=116 , issue=23 , pages=14620–14674 , pmid=27960268 Related compounds *terpyridine Terpyridine (2,2';6',2"-terpyridine, often abbreviated to Terpy or Tpy) is a heterocyclic compound derived from pyridine. It is a white solid that is soluble in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetradentate
In chemistry, tetradentate ligands are ligands that bind four donor atoms to a central atom to form a coordination complex. This number of donor atoms that bind is called denticity and is a method of classifying ligands. Tetradentate ligands are common in nature in the form of chlorophyll, which has a core ligand called chlorin, and heme, which has a core ligand called porphyrin. They are responsible for the colour observed in plants and humans. Phthalocyanine is an artificial macrocyclic tetradentate ligand that is used to make blue and green pigments. Shape Tetradentate ligands can be classified by the topology of the connections between donor atoms. Common forms are linear (also called sequential), ring or tripodal. A tetrapodal ligand that is also tetradentate has four legs with donor atoms and a bridgehead that is not a donor. Upon binding with a central atom, there are several arrangements possible (known as geometric isomers). Linear ligands A linear tetradentate ligand ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyridine
Pyridine is a basic (chemistry), basic heterocyclic compound, heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid with a distinctive, unpleasant fish-like smell. Pyridine is colorless, but older or impure samples can appear yellow, due to the formation of extended, unsaturated polymeric chains, which show significant electrical conductivity. The pyridine ring occurs in many important compounds, including agrochemicals, pharmaceuticals, and vitamins. Historically, pyridine was produced from coal tar. As of 2016, it is synthesized on the scale of about 20,000 tons per year worldwide. Properties Physical properties The molecular electric dipole moment is 2.2 debyes. Pyridine is diamagnetism, diamagnetic and has a Magnetic susceptibility, diamagnetic susceptibility of −48.7 × 10−6 cm3·mol−1. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bipyridine
Bipyridines also known as bipyridyls, dipyridyls, and dipyridines, are a family of chemical compounds with the formula (C5H4N)2, consisting of two pyridyl (C5H4N) rings. Pyridine is an aromatic nitrogen-containing heterocycle. Bipyridines are of significance in pesticides. Six isomers of bipyridine exist, but two are prominent: 2,2′-bipyridine is a popular ligand. 4,4'-Bipyridine is a precursor to the commercial herbicide paraquat. The bipyridines are all colourless solids, which are soluble in organic solvents and slightly soluble in water. 2,2′-Bipyridine 2,2′-Bipyridine (2,2′-bipy) is a chelating ligand that forms complexes with most transition metal ions that are of broad academic interest. Many of these complexes have distinctive optical properties, and some are of interest for analysis. Its complexes are used in studies of electron and energy transfer, supramolecular and materials chemistry, and catalysis. 2,2′-Bipyridine is used in the manufacture of d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2,2'-Bipyridine
The comma is a punctuation mark that appears in several variants in different languages. It has the same shape as an apostrophe or single closing quotation mark () in many typefaces, but it differs from them in being placed on the baseline of the text. Some typefaces render it as a small line, slightly curved or straight, but inclined from the vertical. Other fonts give it the appearance of a miniature filled-in figure on the baseline. The comma is used in many contexts and languages, mainly to separate parts of a sentence such as clauses, and items in lists mainly when there are three or more items listed. The word ''comma'' comes from the Greek (), which originally meant a cut-off piece, specifically in grammar, a short clause. A comma-shaped mark is used as a diacritic in several writing systems and is considered distinct from the cedilla. In Byzantine and modern copies of Ancient Greek, the " rough" and "smooth breathings" () appear above the letter. In Latvian, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ligand
In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs, often through Lewis bases. The nature of metal–ligand bonding can range from covalent to ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acidic "ligands". Metals and metalloids are bound to ligands in almost all circumstances, although gaseous "naked" metal ions can be generated in a high vacuum. Ligands in a complex dictate the reactivity of the central atom, including ligand substitution rates, the reactivity of the ligands themselves, and redox. Ligand selection requires critical consideration in many practical areas, including bioinorganic and medicinal chemistry, homogeneous catalysis, and environm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coordination Chemistry
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of chemical bond, bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many metal-containing chemical compound, compounds, especially those that include transition metals (elements like titanium that belong to the Block (periodic table), Periodic Table's d-block), are coordination complexes. Nomenclature and terminology Coordination complexes are so pervasive that their structures and reactions are described in many ways, sometimes confusingly. The atom within a ligand that is bonded to the central metal atom or ion is called the donor atom. In a typical complex, a metal ion is bonded to several donor atoms, which can be the same or different. A Ligand#Polydentate and polyhapto ligand motifs and nomenclature, polydentate (multiple bonded) ligand is a molecule or ion that bonds to the central atom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Terpyridine
Terpyridine (2,2';6',2"-terpyridine, often abbreviated to Terpy or Tpy) is a heterocyclic compound derived from pyridine. It is a white solid that is soluble in most organic solvents. The compound is mainly used as a ligand in coordination chemistry. Synthesis Terpyridine was first synthesized by G. Morgan and F. H. Burstall in 1932 by the oxidative coupling of pyridines. This method, however, proceeded in low yields. More efficient syntheses have since been described, mainly starting from 2-acetylpyridine. One method produces an enaminone by the reaction of 2-acetylpyridine with N,N-dimethylformamide dimethyl acetal. The base-catalyzed reaction of 2-acetylpyridine with carbon disulfide followed by alkylation with methyl iodide gives C5H4NCOCH=C(SMe)2. Condensation of this species with 2-acetylpyridine forms the related 1,5-diketone, which condenses with ammonium acetate to form a terpyridine. Treatment of this derivative with Raney nickel removes the thioether group. Ot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chelating Agents
Chelation is a type of bonding of ions and molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These ligands are called chelants, chelators, chelating agents, or sequestering agents. They are usually organic compounds, but this is not a necessity, as in the case of zinc and its use as a maintenance therapy to prevent the absorption of copper in people with Wilson's disease. Chelation is useful in applications such as providing nutritional supplements, in chelation therapy to remove toxic metals from the body, as contrast agents in MRI scanning, in manufacturing using homogeneous catalysts, in chemical water treatment to assist in the removal of metals, and in fertilizers. Chelate effect The chelate effect is the greater affinity of chelating ligands for a metal ion than that of similar nonchelating (monodentate) ligands for the same metal. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyridines
Pyridine is a basic heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid with a distinctive, unpleasant fish-like smell. Pyridine is colorless, but older or impure samples can appear yellow, due to the formation of extended, unsaturated polymeric chains, which show significant electrical conductivity. The pyridine ring occurs in many important compounds, including agrochemicals, pharmaceuticals, and vitamins. Historically, pyridine was produced from coal tar. As of 2016, it is synthesized on the scale of about 20,000 tons per year worldwide. Properties Physical properties The molecular electric dipole moment is 2.2 debyes. Pyridine is diamagnetic and has a diamagnetic susceptibility of −48.7 × 10−6 cm3·mol−1. The standard enthalpy of formation is 100.2 kJ·mol−1 in the liquid phas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
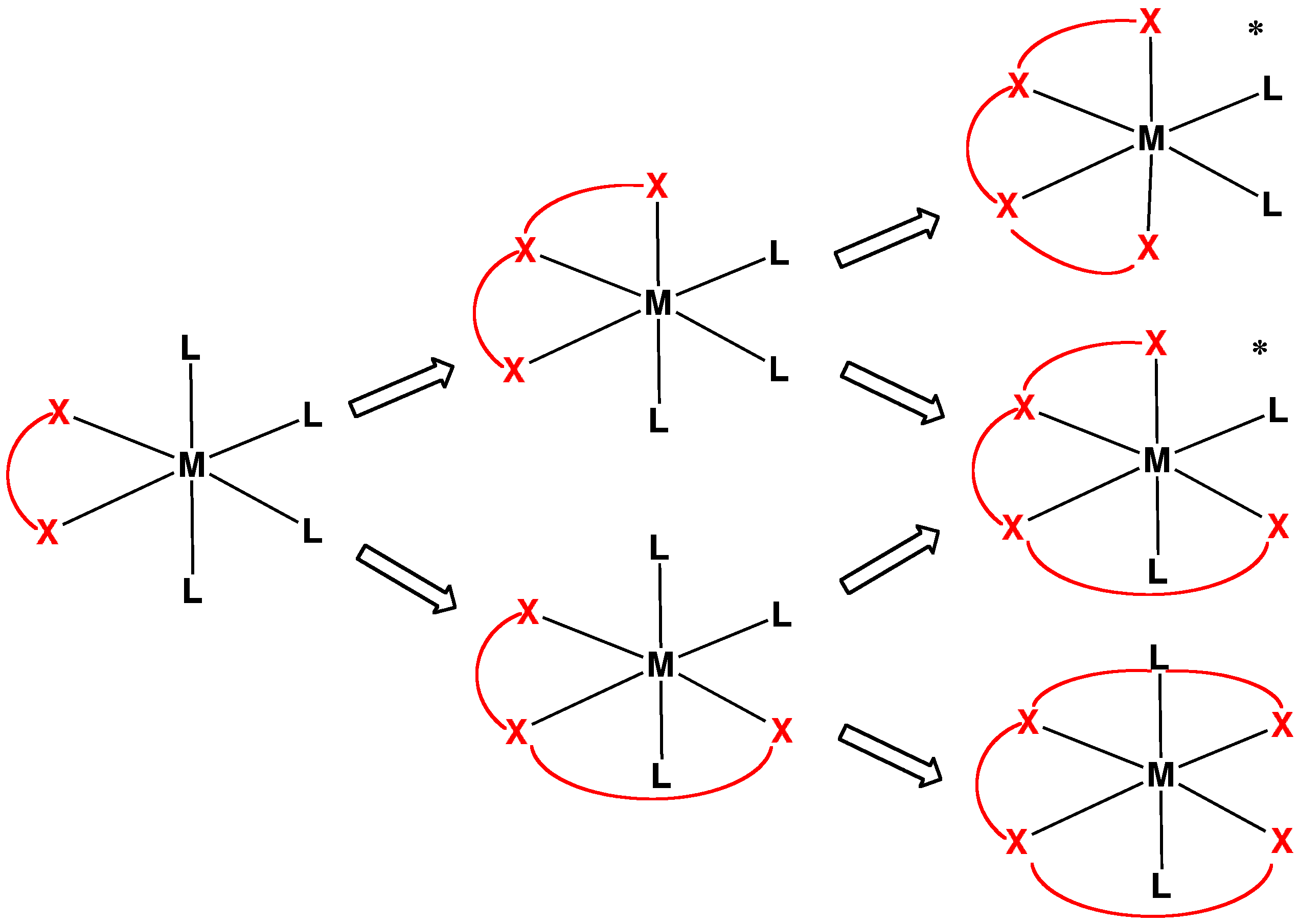
4-3D-balls.png)