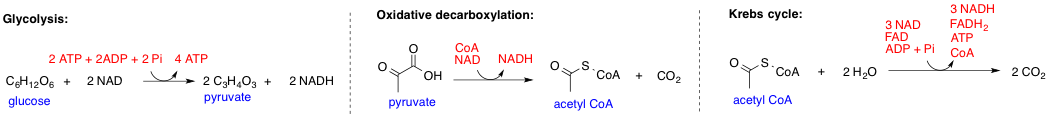|
Pyruvaldehyde
Methylglyoxal (MGO) is the organic compound with the formula CH3C(O)CHO. It is a reduced derivative of pyruvic acid. It is a reactive compound that is implicated in the biology of diabetes. Methylglyoxal is produced industrially by degradation of carbohydrates using overexpressed methylglyoxal synthase. Chemical structure Gaseous methylglyoxal has two carbonyl groups, an aldehyde and a ketone. In the presence of water, it exists as hydrates and oligomers. The formation of these hydrates is indicative of the high reactivity of MGO, which is relevant to its biological behavior. Biochemistry Biosynthesis and biodegradation In organisms, methylglyoxal is formed as a side-product of several metabolic pathways. Methylglyoxal mainly arises as side products of glycolysis involving glyceraldehyde-3-phosphate and dihydroxyacetone phosphate. It is also thought to arise via the degradation of acetone and threonine. Illustrative of the myriad pathways to MGO, aristolochic acid caused 12 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bond C=O). The simplest ketone is acetone (where R and R' is methyl), with the formula . Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids (e.g., testosterone), and the solvent acetone. Nomenclature and etymology The word ''ketone'' is derived from ''Aketon'', an old German word for ''acetone''. According to the rules of IUPAC nomenclature, ketone names are derived by changing the suffix ''-ane'' of the parent alkane to ''-anone''. Typically, the position of the carbonyl group is denoted by a number, but traditional nonsystematic names are still generally used for the most important ketones, for example acetone and benzophenone. These nonsystematic names are considere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metabolic Pathway
In biochemistry, a metabolic pathway is a linked series of chemical reactions occurring within a cell. The reactants, products, and intermediates of an enzymatic reaction are known as metabolites, which are modified by a sequence of chemical reactions catalyzed by enzymes. In most cases of a metabolic pathway, the product of one enzyme acts as the substrate for the next. However, side products are considered waste and removed from the cell. These enzymes often require dietary minerals, vitamins, and other cofactors to function. Different metabolic pathways function based on the position within a eukaryotic cell and the significance of the pathway in the given compartment of the cell. For instance, the, electron transport chain, and oxidative phosphorylation all take place in the mitochondrial membrane. In contrast, glycolysis, pentose phosphate pathway, and fatty acid biosynthesis all occur in the cytosol of a cell. There are two types of metabolic pathways that are character ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lactic Acid
Lactic acid is an organic acid. It has a molecular formula . It is white in the solid state and it is miscible with water. When in the dissolved state, it forms a colorless solution. Production includes both artificial synthesis as well as natural sources. Lactic acid is an alpha-hydroxy acid (AHA) due to the presence of a hydroxyl group adjacent to the carboxyl group. It is used as a synthetic intermediate in many organic synthesis industries and in various biochemical industries. The conjugate base of lactic acid is called lactate (or the lactate anion). The name of the derived acyl group is lactoyl. In solution, it can ionize by loss of a proton to produce the lactate ion . Compared to acetic acid, its p''K'' is 1 unit less, meaning lactic acid is ten times more acidic than acetic acid. This higher acidity is the consequence of the intramolecular hydrogen bonding between the α-hydroxyl and the carboxylate group. Lactic acid is chiral, consisting of two enantiomers. One ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glyoxalase I
The enzyme lactoylglutathione lyase (EC 4.4.1.5, also known as glyoxalase I) catalyzes the isomerization of hemithioacetal adducts, which are formed in a spontaneous reaction between a glutathionyl group and aldehydes such as methylglyoxal. :(''R'')-''S''-lactoylglutathione = glutathione + 2-oxopropanal Glyoxalase I derives its name from its catalysis of the first step in the glyoxalase system, a critical two-step detoxification system for methylglyoxal. Methylglyoxal is produced naturally as a byproduct of normal biochemistry, but is highly toxic, due to its chemical reactions with proteins, nucleic acids, and other cellular components. The second detoxification step, in which (''R'')-''S''-lactoylglutathione is split into glutathione and D-lactate, is carried out by glyoxalase II, a hydrolase. Unusually, these reactions carried out by the glyoxalase system does not oxidize glutathione, which usually acts as a redox coenzyme. Although aldose reductase can also detoxify me ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glutathione
Glutathione (GSH, ) is an antioxidant in plants, animals, fungi, and some bacteria and archaea. Glutathione is capable of preventing damage to important cellular components caused by sources such as reactive oxygen species, free radicals, peroxides, lipid peroxides, and heavy metals. It is a tripeptide with a gamma peptide linkage between the carboxyl group of the glutamate side chain and cysteine. The carboxyl group of the cysteine residue is attached by normal peptide linkage to glycine. Biosynthesis and occurrence Glutathione biosynthesis involves two adenosine triphosphate-dependent steps: *First, γ-glutamylcysteine is synthesized from L- glutamate and cysteine. This conversion requires the enzyme glutamate–cysteine ligase (GCL, glutamate cysteine synthase). This reaction is the rate-limiting step in glutathione synthesis. *Second, glycine is added to the C-terminal of γ-glutamylcysteine. This condensation is catalyzed by glutathione synthetase. While all animal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glyoxalase System
The glyoxalase system is a set of enzymes that carry out the detoxification of methylglyoxal and the other reactive aldehydes that are produced as a normal part of metabolism. This system has been studied in both bacteria and eukaryotes. This detoxification is accomplished by the sequential action of two thiol-dependent enzymes; firstly glyoxalase І, which catalyzes the isomerization of the spontaneously formed hemithioacetal adduct between glutathione and 2-oxoaldehydes (such as methylglyoxal) into S-2-hydroxyacylglutathione. Secondly, glyoxalase ІІ hydrolyses these thiolesters and in the case of methylglyoxal catabolism, produces D-lactate and GSH from S-D-lactoyl-glutathione. This system shows many of the typical features of the enzymes that dispose of endogenous toxins. Firstly, in contrast to the amazing substrate range of many of the enzymes involved in xenobiotic metabolism, it shows a narrow substrate specificity. Secondly, intracellular thiols are required as part of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytotoxic
Cytotoxicity is the quality of being toxic to cells. Examples of toxic agents are an immune cell or some types of venom, e.g. from the puff adder (''Bitis arietans'') or brown recluse spider (''Loxosceles reclusa''). Cell physiology Treating cells with the cytotoxic compound can result in a variety of cell fates. The cells may undergo necrosis, in which they lose membrane integrity and die rapidly as a result of cell lysis. The cells can stop actively growing and dividing (a decrease in cell viability), or the cells can activate a genetic program of controlled cell death (apoptosis). Cells undergoing necrosis typically exhibit rapid swelling, lose membrane integrity, shut down metabolism, and release their contents into the environment. Cells that undergo rapid necrosis in vitro do not have sufficient time or energy to activate apoptotic machinery and will not express apoptotic markers. Apoptosis is characterized by well defined cytological and molecular events including a change i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,2-Propanediol
Propylene glycol (IUPAC name: propane-1,2-diol) is a viscous, colorless liquid, which is nearly odorless but possesses a faintly sweet taste. Its chemical formula is CH3CH(OH)CH2OH. Containing two alcohol groups, it is classed as a diol. It is miscible with a broad range of solvents, including water, acetone, and chloroform. In general, glycols are non-irritating and have very low volatility. It is produced on a large scale primarily for the production of polymers. In the European Union, it has E-number E1520 for food applications. For cosmetics and pharmacology, the number is E490. Propylene glycol is also present in propylene glycol alginate, which is known as E405. Propylene glycol is a compound which is GRAS (generally recognized as safe) by the US Food and Drug Administration under 21 CFR x184.1666, and is also approved by the FDA for certain uses as an indirect food additive. Propylene glycol is approved and used as a vehicle for topical, oral, and some intravenous pharm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lipid Peroxidation
Lipid peroxidation is the chain of reactions of oxidative degradation of lipids. It is the process in which radical (chemistry), free radicals "steal" electrons from the lipids in cell membranes, resulting in cell damage. This process proceeds by a free radical chain reaction#Chemical chain reactions, chain reaction mechanism. It most often affects polyunsaturated fatty acids, because they contain multiple double bonds in between which lie methylene bridges (-CH2-) that possess especially reactive hydrogen atoms. As with any radical reaction, the reaction consists of three major steps: initiation, propagation, and termination. The chemical products of this oxidation are known as lipid peroxides or lipid oxidation products (LOPs). Initiation Initiation is the step in which a fatty acid radical (chemistry), radical is produced. The most notable initiators in living cells are reactive oxygen species (ROS), such as hydroxyl radical, OH· and hydroperoxyl, HOO·, which combines wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Threonine
Threonine (symbol Thr or T) is an amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form under biological conditions), a carboxyl group (which is in the deprotonated −COO− form under biological conditions), and a side chain containing a hydroxyl group, making it a polar, uncharged amino acid. It is essential in humans, meaning the body cannot synthesize it: it must be obtained from the diet. Threonine is synthesized from aspartate in bacteria such as ''E. coli''. It is encoded by all the codons starting AC (ACU, ACC, ACA, and ACG). Threonine sidechains are often hydrogen bonded; the most common small motifs formed are based on interactions with serine: ST turns, ST motifs (often at the beginning of alpha helices) and ST staples (usually at the middle of alpha helices). Modifications The threonine residue is susceptible to numerous posttranslational modifications. The hydroxyl side-chain can unde ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3-aminoacetone
Aminoacetone is the simplest monopeptide with the formula CH3C(O)CH2NH2. Although stable in the gaseous form, once condensed it reacts with itself. The protonated derivative forms stable salts, e.g. aminoacetone hydrochloride ( H3C(O)CH2NH3l)). The semicarbazone of the hydrochloride is another bench-stable precursor. Aminoacetone is a metabolite that is implicated in the biosynthesis of methylglyoxal. See also * Propanolamines * Aminoaldehydes and aminoketones Aminoaldehydes and aminoketones are organic compounds that contain an amine functional group as well as either a aldehyde or ketone functional group. These compounds are important in biology and in chemical synthesis. Because of their bifunctional ... References Amines Ketones {{amine-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aristolochic Acid
Aristolochic acids () are a family of carcinogenic, mutagenic, and nephrotoxic phytochemicals commonly found in the flowering plant family Aristolochiaceae (birthworts). Aristolochic acid (AA) I is the most abundant one. The family Aristolochiaceae includes the genera ''Aristolochia'' and '' Asarum'' (wild ginger), which are commonly used in Chinese herbal medicine. Although these compounds are widely associated with kidney problems, liver and urothelial cancers, the use of AA-containing plants for medicinal purposes has a long history. The FDA has issued warnings regarding consumption of AA-containing supplements. History Early medical uses Birthwort plants, and the aristolochic acids they contain, were quite common in ancient Greek and Roman medical texts, well-established as an herb there by the fifth century BC. Birthworts appeared in Ayurvedic texts by 400 AD, and in Chinese texts later in the fifth century. In these ancient times, it was used to treat kidney and urinary pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |