|
Prion
Prions are misfolded proteins that have the ability to transmit their misfolded shape onto normal variants of the same protein. They characterize several fatal and transmissible neurodegenerative diseases in humans and many other animals. It is not known what causes a normal protein to misfold, but the resulting abnormal three-dimensional structure confers infectious properties by collapsing nearby protein molecules into the same shape. The word ''prion'' is derived from the term, "proteinaceous infectious particle". In comparison to all other known infectious agents such as viroids, viruses, bacteria, fungi, and parasites, all of which contain nucleic acids ( DNA, RNA, or both), the hypothesized role of a protein as an infectious agent stands in contrast. Prion isoforms of the prion protein (PrP), whose specific function is uncertain, are hypothesized as the cause of transmissible spongiform encephalopathies (TSEs), including scrapie in sheep, chronic wasting disease (CWD) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Creutzfeldt–Jakob Disease
Creutzfeldt–Jakob disease (CJD), also known as subacute spongiform encephalopathy or neurocognitive disorder due to prion disease, is an invariably fatal degenerative brain disorder. Early symptoms include memory problems, behavioral changes, poor coordination, and visual disturbances. Later symptoms include dementia, involuntary movements, blindness, weakness, and coma. About 70% of people die within a year of diagnosis. The name Creutzfeldt–Jakob disease was introduced by Walther Spielmeyer in 1922, after the German neurologists Hans Gerhard Creutzfeldt and Alfons Maria Jakob. CJD is caused by a type of abnormal protein known as a prion. Infectious prions are misfolded proteins that can cause normally folded proteins to also become misfolded. About 85% of cases of CJD occur for unknown reasons, while about 7.5% of cases are inherited in an autosomal dominant manner. Exposure to brain or spinal tissue from an infected person may also result in spread. There is no evid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
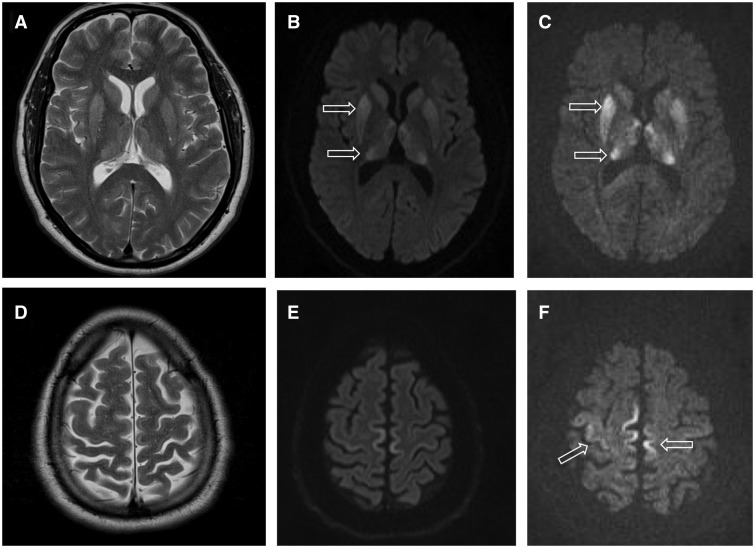
Transmissible Spongiform Encephalopathies
Transmissible spongiform encephalopathies (TSEs) are a group of progressive and fatal conditions that are associated with prions and affect the brain and nervous system of many animals, including humans, cattle, and sheep. According to the most widespread hypothesis, they are transmitted by prions, though some other data suggest an involvement of a ''Spiroplasma'' infection. Mental and physical abilities deteriorate and many tiny holes appear in the cortex causing it to appear like a sponge when brain tissue obtained at autopsy is examined under a microscope. The disorders cause impairment of brain function, including memory changes, personality changes and problems with movement that worsen chronically. TSEs of humans include Creutzfeldt–Jakob disease, Gerstmann–Sträussler–Scheinker syndrome, fatal familial insomnia, and kuru, as well as the recently discovered variably protease-sensitive prionopathy and familial spongiform encephalopathy. Creutzfeldt-Jakob disease itsel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Scrapie
Scrapie () is a fatal, degenerative disease affecting the nervous systems of sheep and goats. It is one of several transmissible spongiform encephalopathies (TSEs), and as such it is thought to be caused by a prion. Scrapie has been known since at least 1732 and does not appear to be transmissible to humans. However, new studies suggest a link between scrapie and sporadic CJD. The name scrapie is derived from one of the clinical signs of the condition, wherein affected animals will compulsively scrape off their fleeces against rocks, trees or fences. The disease apparently causes an itching sensation in the animals. Other clinical signs include excessive lip smacking, altered gaits and convulsive collapse. Scrapie is infectious and transmissible among conspecifics, so one of the most common ways to contain it (since it is incurable) is to quarantine and kill those affected. However, scrapie tends to persist in flocks and can also arise apparently spontaneously in flocks that ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bovine Spongiform Encephalopathy
Bovine spongiform encephalopathy (BSE), commonly known as mad cow disease, is an incurable and invariably fatal neurodegenerative disease of cattle. Symptoms include abnormal behavior, trouble walking, and weight loss. Later in the course of the disease the cow becomes unable to function normally. There is conflicting information around the time between infection and onset of symptoms. In 2002, the WHO suggested it to be approximately four to five years. Time from onset of symptoms to death is generally weeks to months. Spread to humans is believed to result in variant Creutzfeldt–Jakob disease (vCJD). As of 2018, a total of 231 cases of vCJD had been reported globally. BSE is thought to be due to an infection by a misfolded protein, known as a prion. Cattle are believed to have been infected by being fed meat-and-bone meal (MBM) that contained either the remains of cattle who spontaneously developed the disease or scrapie-infected sheep products. The outbreak increased th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chronic Wasting Disease
Chronic wasting disease (CWD), sometimes called zombie deer disease, is a transmissible spongiform encephalopathy (TSE) affecting deer. TSEs are a family of diseases thought to be caused by misfolded proteins called prions and include similar diseases such as BSE (mad cow disease) in cattle, Creutzfeldt-Jakob disease (CJD) in humans and scrapie in sheep. In the United States, CWD affects mule deer, white-tailed deer, red deer, sika deer, elk, caribou, and moose. Natural infection causing CWD affects members of the deer family. Experimental transmission of CWD to other species such as squirrel monkeys and genetically modified mice has been shown. In 1967, CWD was first identified in mule deer at a government research facility in northern Colorado, United States. It was initially recognized as a clinical "wasting" syndrome and then in 1978, it was identified more specifically as a TSE disease. Since then, CWD has been found in free-ranging and captive animal populations in 30 US ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteopathy
In medicine, proteinopathy (; 'pref''. protein -pathy 'suff''. disease proteinopathies ''pl''.; proteinopathic ''adj''), or proteopathy, protein conformational disorder, or protein misfolding disease refers to a class of diseases in which certain proteins become structurally abnormal, and thereby disrupt the function of cells, tissues and organs of the body. Often the proteins fail to fold into their normal configuration; in this misfolded state, the proteins can become toxic in some way (a toxic gain-of-function Gain-of-function research (GoF research or GoFR) is medical research that genetically alters an organism in a way that may enhance the biological functions of gene products. This may include an altered pathogenesis, transmissibility, or host ...) or they can lose their normal function. The proteinopathies include such diseases as Creutzfeldt–Jakob disease and other prion, prion diseases, Alzheimer's disease, Parkinson's disease, amyloidosis, multiple system ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteinopathy
In medicine, proteinopathy (; 'pref''. protein -pathy 'suff''. disease proteinopathies ''pl''.; proteinopathic ''adj''), or proteopathy, protein conformational disorder, or protein misfolding disease refers to a class of diseases in which certain proteins become structurally abnormal, and thereby disrupt the function of cells, tissues and organs of the body. Often the proteins fail to fold into their normal configuration; in this misfolded state, the proteins can become toxic in some way (a toxic gain-of-function) or they can lose their normal function. The proteinopathies include such diseases as Creutzfeldt–Jakob disease and other prion diseases, Alzheimer's disease, Parkinson's disease, amyloidosis, multiple system atrophy, and a wide range of other disorders. The term ''proteopathy'' was first proposed in 2000 by Lary Walker and Harry LeVine. The concept of proteopathy can trace its origins to the mid-19th century, when, in 1854, Rudolf Virchow coined the term amyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
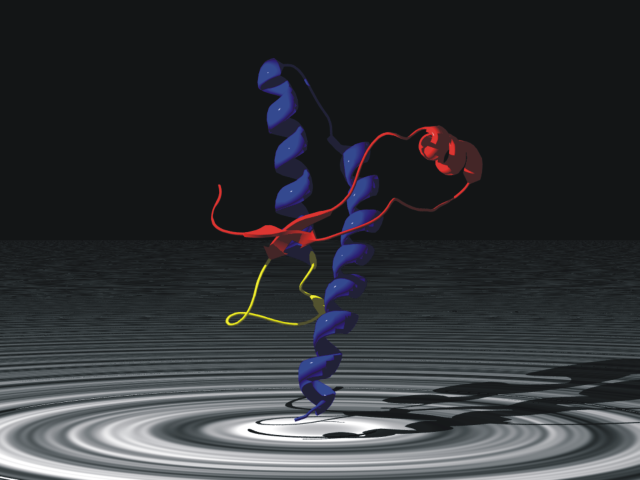
Prion Protein
Major prion protein (PrP), is encoded in the human by the ''PRNP'' gene also known as CD230 (cluster of differentiation 230). Expression of the protein is most predominant in the nervous system but occurs in many other tissues throughout the body. The protein can exist in multiple isoforms: the normal PrPC form, and the protease-resistant form designated PrPRes such as the disease-causing PrPSc(scrapie) and an isoform located in mitochondria. The misfolded version PrPSc is associated with a variety of cognitive disorders and neurodegenerative diseases such as in animals: ovine scrapie, bovine spongiform encephalopathy (BSE, mad cow disease), feline spongiform encephalopathy, transmissible mink encephalopathy (TME), exotic ungulate encephalopathy, chronic wasting disease (CWD) which affects cervids; and in humans: Creutzfeldt–Jakob disease (CJD), fatal familial insomnia (FFI), Gerstmann–Sträussler–Scheinker syndrome (GSS), kuru, and variant Creutzfeldt–Jakob disease (v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
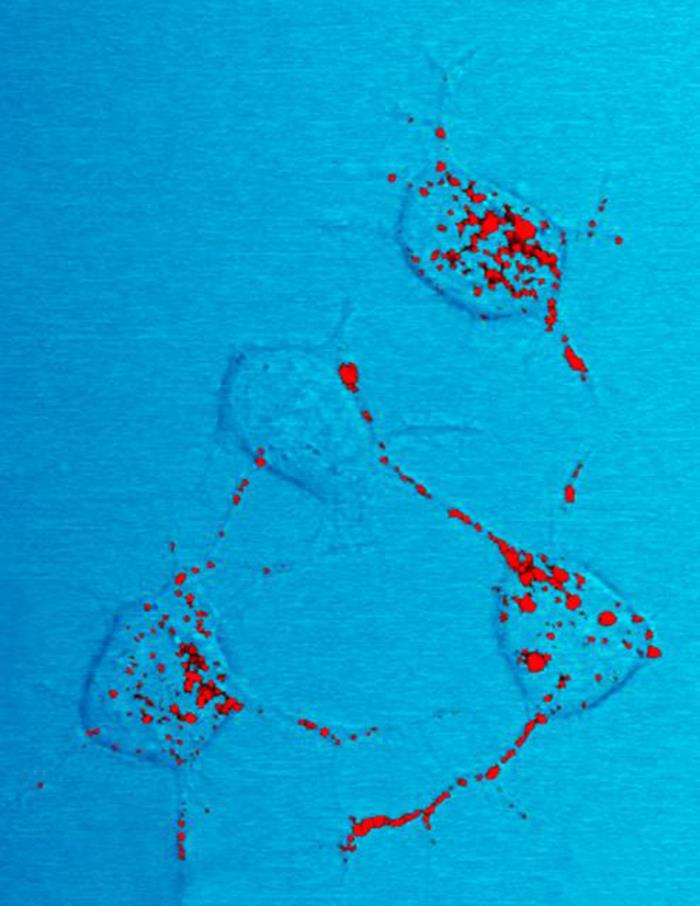
Amyloids
Amyloids are aggregates of proteins characterised by a fibrillar morphology of 7–13 nm in diameter, a beta sheet (β-sheet) secondary structure (known as cross-β) and ability to be stained by particular dyes, such as Congo red. In the human body, amyloids have been linked to the development of various diseases. Pathogenic amyloids form when previously healthy proteins lose their normal structure and physiological functions (misfolding) and form fibrous deposits in amyloid plaques around cells which can disrupt the healthy function of tissues and organs. Such amyloids have been associated with (but not necessarily as the cause of) more than 50 human diseases, known as amyloidosis, and may play a role in some neurodegenerative diseases. Some of these diseases are mainly sporadic and only a few cases are familial. Others are only familial. Some are iatrogenic as they result from medical treatment. Prions are an infectious form of amyloids that can act as a template to conv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Viroid
Viroids are small single-stranded, circular RNAs that are infectious pathogens. Unlike viruses, they have no protein coating. All known viroids are inhabitants of angiosperms (flowering plants), and most cause diseases, whose respective economic importance to humans varies widely. The first discoveries of viroids in the 1970s triggered the historically third major extension of the biosphere—to include smaller lifelike entities —after the discoveries in 1675 by Antonie van Leeuwenhoek (of the "subvisible" microorganisms) and in 1892–1898 by Dmitri Iosifovich Ivanovsky and Martinus Beijerinck (of the "submicroscopic" viruses). The unique properties of viroids have been recognized by the International Committee on Taxonomy of Viruses, in creating a new order of subviral agents. The first recognized viroid, the pathogenic agent of the potato spindle tuber disease, was discovered, initially molecularly characterized, and named by Theodor Otto Diener, plant pathologist a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha-synuclein
Alpha-synuclein is a protein that, in humans, is encoded by the ''SNCA'' gene. Alpha-synuclein is a neuronal protein that regulates synaptic vesicle trafficking and subsequent neurotransmitter release. It is abundant in the brain, while smaller amounts are found in the heart, muscle and other tissues. In the brain, alpha-synuclein is found mainly in the axon terminals of presynaptic neurons. Within these terminals, alpha-synuclein interacts with phospholipids and proteins. Presynaptic terminals release chemical messengers, called neurotransmitters, from compartments known as synaptic vesicles. The release of neurotransmitters relays signals between neurons and is critical for normal brain function. The human alpha-synuclein protein is made of 140 amino acids. An alpha-synuclein fragment, known as the non- Abeta component (NAC) of Alzheimer's disease amyloid, originally found in an amyloid-enriched fraction, was shown to be a fragment of its precursor protein, NACP. It was later de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neurodegenerative Diseases
A neurodegenerative disease is caused by the progressive loss of structure or function of neurons, in the process known as neurodegeneration. Such neuronal damage may ultimately involve cell death. Neurodegenerative diseases include amyotrophic lateral sclerosis, multiple sclerosis, Parkinson's disease, Alzheimer's disease, Huntington's disease, multiple system atrophy, and prion diseases. Neurodegeneration can be found in the brain at many different levels of neuronal circuitry, ranging from molecular to systemic. Because there is no known way to reverse the progressive degeneration of neurons, these diseases are considered to be incurable; however research has shown that the two major contributing factors to neurodegeneration are oxidative stress and inflammation. Biomedical research has revealed many similarities between these diseases at the subcellular level, including atypical protein assemblies (like proteinopathy) and induced cell death. These similarities suggest that th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |