|
Polyhalide
Polyhalogen ions are a group of polyatomic cations and anions containing halogens only. The ions can be classified into two classes, isopolyhalogen ions which contain one type of halogen only, and heteropolyhalogen ions with more than one type of halogen. Introduction Numerous polyhalogen ions have been found, with their salts isolated in the solid state and structurally characterized. The following tables summarize the known species. can only exist as at low temperatures, a charge-transfer complex from to . Free is only known from its electronic band spectrum obtained in a low-pressure discharge tube. The existence of is possible but still uncertain. Structure Most of the structures of the ions have been determined by IR spectroscopy, Raman spectroscopy and X-ray crystallography. The polyhalogen ions always have the heaviest and least electronegative halogen present in the ion as the central atom, making the ion asymmetric in some cases. For exa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyiodide
The polyiodides are a class of polyhalogen anions composed entirely of iodine atoms. The most common and simplest member is the triiodide ion, . Other known larger polyiodides include 4sup>2−, 5sup>−, 6sup>2−, 7sup>−, 8sup>2−, 9sup>−, 10sup>2−, 10sup>4−, 11sup>3−, 12sup>2−, 13sup>3−, 14sup>4-, 16sup>2−, 22sup>4−, 26sup>3−, 26sup>4−, 28sup>4− and 29sup>3−. All these can be considered as formed from the interaction of the I–, I2, and building blocks. Preparation The polyiodides can be made by addition of stoichiometric amounts of I2 to solutions containing I− and , with the presence of large countercations to stabilize them. For example, KI3·H2O can be crystallized from a saturated solution of KI when a stoichiometric amount of I2 is added and cooled. Structure ] ] Polyiodides are characterized by their highly complex and variable structures, and can be considered as associations of I2, I−, and units. Discrete polyiodid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cation
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons while an anion is a negatively charged ion with more electrons than protons. Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed atomic or monatomic ions, while two or more atoms form molecular ions or polyatomic ions. In the case of physical ionization in a fluid (gas or liquid), "ion pairs" are created by spontaneous molecule collisions, where each generated pair consists of a free electron and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wavenumbers
In the physical sciences, the wavenumber (also wave number or repetency) is the ''spatial frequency'' of a wave, measured in cycles per unit distance (ordinary wavenumber) or radians per unit distance (angular wavenumber). It is analogous to temporal frequency, which is defined as the number of wave cycles per unit time (''ordinary frequency'') or radians per unit time (''angular frequency''). In multidimensional systems, the wavenumber is the magnitude of the ''wave vector''. The space of wave vectors is called ''reciprocal space''. Wave numbers and wave vectors play an essential role in optics and the physics of wave scattering, such as X-ray diffraction, neutron diffraction, electron diffraction, and elementary particle physics. For quantum mechanical waves, the wavenumber multiplied by the reduced Planck's constant is the ''canonical momentum''. Wavenumber can be used to specify quantities other than spatial frequency. For example, in optical spectroscopy, it is often use ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodine Monochloride
Iodine monochloride is an interhalogen compound with the formula . It is a red-brown chemical compound that melts near room temperature. Because of the difference in the electronegativity of iodine and chlorine, this molecule is highly polar and behaves as a source of I+. Preparation Iodine monochloride is produced simply by combining the halogens in a 1:1 molar ratio, according to the equation : When chlorine gas is passed through iodine crystals, one observes the brown vapor of iodine monochloride. Dark brown iodine monochloride liquid is collected. Excess chlorine converts iodine monochloride into iodine trichloride in a reversible reaction: : Polymorphs has two polymorphs; α-ICl, which exists as black needles (red by transmitted light) with a melting point of 27.2 °C, and β-ICl, which exists as black platelets (red-brown by transmitted light) with a melting point 13.9 °C.Brisbois, R. G.; Wanke, R. A.; Stubbs, K. A.; Stick, R. V. "Iodine Monochloride" ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Canonical Forms Of (ICl2)-
The adjective canonical is applied in many contexts to mean "according to the canon" the standard, rule or primary source that is accepted as authoritative for the body of knowledge or literature in that context. In mathematics, "canonical example" is often used to mean "archetype". Science and technology * Canonical form, a natural unique representation of an object, or a preferred notation for some object Mathematics * * Canonical coordinates, sets of coordinates that can be used to describe a physical system at any given point in time * Canonical map, a morphism that is uniquely defined by its main property * Canonical polyhedron, a polyhedron whose edges are all tangent to a common sphere, whose center is the average of its vertices * Canonical ring, a graded ring associated to an algebraic variety * Canonical injection, in set theory * Canonical representative, in set theory a standard member of each element of a set partition Differential geometry * Canonical one-form, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
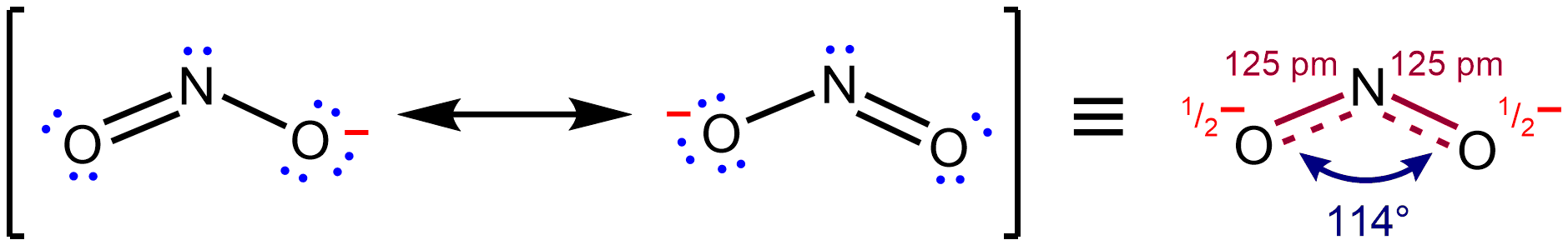
Resonance Hybrid
In chemistry, resonance, also called mesomerism, is a way of describing bonding in certain molecules or polyatomic ions by the combination of several contributing structures (or ''forms'', also variously known as ''resonance structures'' or ''canonical structures'') into a resonance hybrid (or ''hybrid structure'') in valence bond theory. It has particular value for analyzing delocalized electrons where the bonding cannot be expressed by one single Lewis structure. Overview Under the framework of valence bond theory, resonance is an extension of the idea that the bonding in a chemical species can be described by a Lewis structure. For many chemical species, a single Lewis structure, consisting of atoms obeying the octet rule, possibly bearing formal charges, and connected by bonds of positive integer order, is sufficient for describing the chemical bonding and rationalizing experimentally determined molecular properties like bond lengths, angles, and dipole moment. However ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Resonance (chemistry)
In chemistry, resonance, also called mesomerism, is a way of describing bonding in certain molecules or polyatomic ions by the combination of several contributing structures (or ''forms'', also variously known as ''resonance structures'' or ''canonical structures'') into a resonance hybrid (or ''hybrid structure'') in valence bond theory. It has particular value for analyzing delocalized electrons where the bonding cannot be expressed by one single Lewis structure. Overview Under the framework of valence bond theory, resonance is an extension of the idea that the bonding in a chemical species can be described by a Lewis structure. For many chemical species, a single Lewis structure, consisting of atoms obeying the octet rule, possibly bearing formal charges, and connected by bonds of positive integer order, is sufficient for describing the chemical bonding and rationalizing experimentally determined molecular properties like bond lengths, angles, and dipole moment. Howev ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bond Strength
In chemistry, bond energy (''BE''), also called the mean bond enthalpy or average bond enthalpy is the measure of bond strength in a chemical bond. IUPAC defines bond energy as the average value of the gas-phase bond-dissociation energy (usually at a temperature of 298.15 K) for all bonds of the same type within the same chemical species. The bond dissociation energy (enthalpy) is also referred to as bond disruption energy, bond energy, bond strength, or binding energy (abbreviation: ''BDE'', ''BE'', or ''D''). It is defined as the standard enthalpy change of the following fission: R - ''X'' → R + ''X''. The ''BDE'', denoted by Dº(R - ''X''), is usually derived by the thermochemical equation, : \begin \mathrmX) \ = \Delta H^\circ_f\mathrm + \Delta H^\circ_f(X) - \Delta H^\circ_f(\mathrmX) \end The enthalpy of formation Δ''Hf''º of a large number of atoms, free radicals, ions, clusters and compounds is available from the websites of NIST, NASA, CODATA, and IUPAC. Most au ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bond Order
In chemistry, bond order, as introduced by Linus Pauling, is defined as the difference between the number of bonds and anti-bonds. The bond order itself is the number of electron pairs (covalent bonds) between two atoms. For example, in diatomic nitrogen N≡N, the bond order between the two nitrogen atoms is 3 (triple bond). In acetylene H–C≡C–H, the bond order between the two carbon atoms is also 3, and the C–H bond order is 1 ( single bond). In carbon monoxide , the bond order between carbon and oxygen is 3. In thiazyl trifluoride , the bond order between sulfur and nitrogen is 3, and between sulfur and fluorine is 1. In diatomic oxygen O=O the bond order is 2 (double bond). In ethylene the bond order between the two carbon atoms is also 2. The bond order between carbon and oxygen in carbon dioxide O=C=O is also 2. In phosgene , the bond order between carbon and oxygen is 2, and between carbon and chlorine is 1. In some molecules, bond orders can be 4 (quadr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antibonding Orbital
In chemical bonding theory, an antibonding orbital is a type of molecular orbital that weakens the chemical bond between two atoms and helps to raise the energy of the molecule relative to the separated atoms. Such an orbital has one or more nodes in the bonding region between the nuclei. The density of the electrons in the orbital is concentrated outside the bonding region and acts to pull one nucleus away from the other and tends to cause mutual repulsion between the two atoms. This is in contrast to a bonding molecular orbital, which has a lower energy than that of the separate atoms, and is responsible for chemical bonds. Diatomic molecules Antibonding molecular orbitals (MOs) are normally ''higher'' in energy than bonding molecular orbitals. Bonding and antibonding orbitals form when atoms combine into molecules. If two hydrogen atoms are initially far apart, they have identical atomic orbitals. However, as the spacing between the two atoms becomes smaller, the electron w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mössbauer Spectroscopy
Mössbauer spectroscopy is a spectroscopic technique based on the Mössbauer effect. This effect, discovered by Rudolf Mössbauer (sometimes written "Moessbauer", German: "Mößbauer") in 1958, consists of the nearly recoil-free emission and absorption of nuclear gamma rays in solids. The consequent nuclear spectroscopy method is exquisitely sensitive to small changes in the chemical environment of certain nuclei. Typically, three types of nuclear interactions may be observed: the isomer shift due to differences in nearby electron densities (also called the chemical shift in older literature), quadrupole splitting due to atomic-scale electric field gradients; and magnetic Zeeman splitting due to non-nuclear magnetic fields. Due to the high energy and extremely narrow line widths of nuclear gamma rays, Mössbauer spectroscopy is a highly sensitive technique in terms of energy (and hence frequency) resolution, capable of detecting changes of just a few parts in 1011. It is a me ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |