|
Plastic Crystallinity
A plastic crystal is a crystal composed of weakly interacting molecules that possess some orientational or conformational degree of freedom. The name plastic crystal refers to the mechanical softness of such phases: they resemble waxes and are easily deformed. If the internal degree of freedom is molecular rotation, the name rotor phase or rotatory phase is also used. Typical examples are the modifications Methane I and Ethane I. In addition to the conventional molecular plastic crystals, there are also emerging ionic plastic crystals, particularly organic ionic plastic crystals (OIPCs) and protic organic ionic plastic crystals (POIPCs). POIPCs are solid protic organic salts formed by proton transfer from a Brønsted acid to a Brønsted base and in essence are protic ionic liquids in the molten state, have found to be promising solid-state proton conductors for high temperature proton-exchange membrane fuel cells. Examples include 1,2,4- triazolium perfluorobutanesulfonate and imid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macroscopic single crystals are usually identifiable by their geometrical shape, consisting of flat faces with specific, characteristic orientations. The scientific study of crystals and crystal formation is known as crystallography. The process of crystal formation via mechanisms of crystal growth is called crystallization or solidification. The word ''crystal'' derives from the Ancient Greek word (), meaning both "ice" and "rock crystal", from (), "icy cold, frost". Examples of large crystals include snowflakes, diamonds, and table salt. Most inorganic solids are not crystals but polycrystals, i.e. many microscopic crystals fused together into a single solid. Polycrystals include most metals, rocks, ceramics, and ice. A third category of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Energy & Environmental Science
''Energy & Environmental Science'' is a monthly peer-reviewed scientific journal publishing original (primary) research and review articles. The journal covers work of an interdisciplinary nature in the biochemical and biophysical sciences and chemical and mechanical engineering disciplines. It covers energy area. ''Energy & Environmental Science'' is published by the Royal Society of Chemistry and the editor-in-chief is Joseph Hupp (Northwestern University, USA). According to the ''Journal Citation Reports'', the journal has a 2017 impact factor of 30.067, ranking it 4th out of 163 journals in the category "Chemistry, Multidisciplinary", first out of 88 journals in the category "Energy & Fuels", first out of 135 journals in the category "Engineering, Chemical", and first among 225 journals in the category "Environmental Sciences". Article types ''Energy & Environmental Science'' publishes the following types of articles: Research Papers (original scientific work); Review Artic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Physical Quantities
A physical quantity is a physical property of a material or system that can be quantified by measurement. A physical quantity can be expressed as a ''value'', which is the algebraic multiplication of a ' Numerical value ' and a ' Unit '. For example, the physical quantity of mass can be quantified as '32.3 kg ', where '32.3' is the numerical value and 'kg' is the Unit. A physical quantity possesses at least two characteristics in common. # Numerical magnitude. # Units Symbols and nomenclature International recommendations for the use of symbols for quantities are set out in ISO/IEC 80000, the IUPAP red book and the Quantities, Units and Symbols in Physical Chemistry, IUPAC green book. For example, the recommended symbol for the physical quantity ''mass'' is ''m'', and the recommended symbol for the quantity ''electric charge'' is ''Q''. Subscripts and indices Subscripts are used for two reasons, to simply attach a name to the quantity or associate it with another quanti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystals
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macroscopic single crystals are usually identifiable by their geometrical shape, consisting of flat faces with specific, characteristic orientations. The scientific study of crystals and crystal formation is known as crystallography. The process of crystal formation via mechanisms of crystal growth is called crystallization or solidification. The word ''crystal'' derives from the Ancient Greek word (), meaning both "ice" and "rock crystal", from (), "icy cold, frost". Examples of large crystals include snowflakes, diamonds, and table salt. Most inorganic solids are not crystals but polycrystals, i.e. many microscopic crystals fused together into a single solid. Polycrystals include most metals, rocks, ceramics, and ice. A third category of s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perfluorocyclohexane
Perfluorocyclohexane or dodecafluorocyclohexane is a chemical which belongs to the class of fluorocarbons, sometimes referred to as perfluorocarbons or PFCs. Fluorocarbons and their derivatives are useful fluoropolymers, refrigerants, solvents, and anesthetics. Synthesis Perfluorocyclohexane can be synthesized by fluorination of cyclohexane. Properties Perfluorocyclohexane is chemically inert and thermally stable. It is a relatively non-toxic, clear, waxy solid, which has a high vapor pressure Vapor pressure (or vapour pressure in English-speaking countries other than the US; see spelling differences) or equilibrium vapor pressure is defined as the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phas ... and therefore sublimes readily at room temperature. In common with other cyclic perfluorocarbons, perfluoromethylcyclohexane can be detected at extremely low concentrations, making it ideal as a tracer. The molecule predominantly exists in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bulletin Des Sociétés Chimiques Belges
The ''Bulletin des Sociétés Chimiques Belges'' (, CODEN BSCBAG) is the Belgium peer-reviewed scientific journal in chemistry. Originally it started under the name * ''Bulletin de l'Association Belge des Chimistes'' ol. 1 (1887/88) to Vol. 17 (1903) but with Vol. 18 (1904) the title name changed. The journal is also known under the title * ''Bulletin de la Société Chimiques de Belgique''. In 1998 this journal was absorbed by the ''European Journal of Organic Chemistry'' and the ''European Journal of Inorganic Chemistry''. See also * Anales de Química * Chemische Berichte * Bulletin de la Société Chimique de France * European Journal of Organic Chemistry * European Journal of Inorganic Chemistry * Gazzetta Chimica Italiana * Liebigs Annalen * Recueil des Travaux Chimiques des Pays-Bas The ''Recueil des Travaux Chimiques des Pays-Bas'' was the Dutch scientific journal for chemistry. It was established in 1882, but from 1897 (vol. 16) to 1919 (vol 38) it was publis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Melting Entropy
Melting, or fusion, is a physical process that results in the phase transition of a substance from a solid to a liquid. This occurs when the internal energy of the solid increases, typically by the application of heat or pressure, which increases the substance's temperature to the melting point. At the melting point, the ordering of ions or molecules in the solid breaks down to a less ordered state, and the solid "melts" to become a liquid. Substances in the molten state generally have reduced viscosity as the temperature increases. An exception to this principle is the element sulfur, whose viscosity increases in the range of 160 °C to 180 °C due to polymerization. Some organic compounds melt through mesophases, states of partial order between solid and liquid. First order phase transition From a thermodynamics point of view, at the melting point the change in Gibbs free energy ''∆G'' of the substances is zero, but there are non-zero changes in the enthal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
X-ray Diffraction
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles and intensities of these diffracted beams, a crystallographer can produce a three-dimensional picture of the density of electrons within the crystal. From this electron density, the mean positions of the atoms in the crystal can be determined, as well as their chemical bonds, their crystallographic disorder, and various other information. Since many materials can form crystals—such as salts, metals, minerals, semiconductors, as well as various inorganic, organic, and biological molecules—X-ray crystallography has been fundamental in the development of many scientific fields. In its first decades of use, this method determined the size of atoms, the lengths and types of chemical bonds, and the atomic-scale differences among various mat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
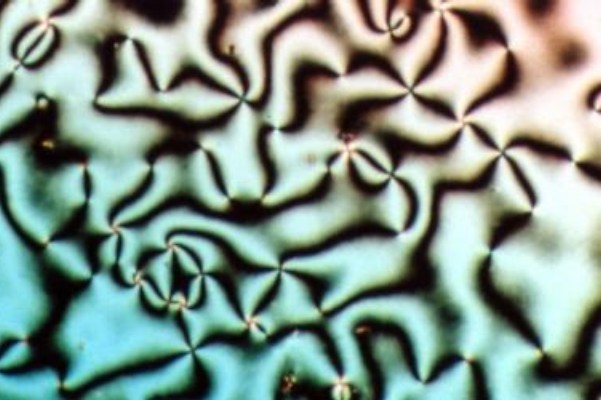
Liquid Crystal
Liquid crystal (LC) is a state of matter whose properties are between those of conventional liquids and those of solid crystals. For example, a liquid crystal may flow like a liquid, but its molecules may be oriented in a crystal-like way. There are many types of LC phases, which can be distinguished by their optical properties (such as textures). The contrasting textures arise due to molecules within one area of material ("domain") being oriented in the same direction but different areas having different orientations. LC materials may not always be in a LC state of matter (just as water may be ice or water vapor). Liquid crystals can be divided into 3 main types: * thermotropic, *lyotropic, and * metallotropic. Thermotropic and lyotropic liquid crystals consist mostly of organic molecules, although a few minerals are also known. Thermotropic LCs exhibit a phase transition into the LC phase as temperature changes. Lyotropic LCs exhibit phase transitions as a function of b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Angewandte Chemie International Edition
''Angewandte Chemie'' (, meaning "Applied Chemistry") is a weekly peer-reviewed scientific journal that is published by Wiley-VCH on behalf of the German Chemical Society (Gesellschaft Deutscher Chemiker). Publishing formats include feature-length reviews, short highlights, research communications, minireviews, essays, book reviews, meeting reviews, correspondences, corrections, and obituaries. This journal contains review articles covering all aspects of chemistry. According to the ''Journal Citation Reports'', the journal had a 2021 impact factor of 16.823. Editions The journal appears in two editions with separate volume and page numbering: a German edition, ''Angewandte Chemie'' ( (print), (online)), and a fully English-language edition, ''Angewandte Chemie International Edition'' ( (print), (online)). The editions are identical in content with the exception of occasional reviews of German-language books or German translations of IUPAC recommendations. Business model ''A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diffraction
Diffraction is defined as the interference or bending of waves around the corners of an obstacle or through an aperture into the region of geometrical shadow of the obstacle/aperture. The diffracting object or aperture effectively becomes a secondary source of the propagating wave. Italian scientist Francesco Maria Grimaldi coined the word ''diffraction'' and was the first to record accurate observations of the phenomenon in 1660. In classical physics, the diffraction phenomenon is described by the Huygens–Fresnel principle that treats each point in a propagating wavefront as a collection of individual spherical wavelets. The characteristic bending pattern is most pronounced when a wave from a coherent source (such as a laser) encounters a slit/aperture that is comparable in size to its wavelength, as shown in the inserted image. This is due to the addition, or interference, of different points on the wavefront (or, equivalently, each wavelet) that travel by paths of d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymorphism (materials Science)
In materials science, polymorphism describes the existence of a solid material in more than one form or crystal structure. Polymorphism is a form of isomerism. Any crystalline material can exhibit the phenomenon. Allotropy refers to polymorphism for chemical elements. Polymorphism is of practical relevance to pharmaceuticals, agrochemicals, pigments, dyestuffs, foods, and explosives. According to IUPAC, a polymorphic transition is "A reversible transition of a solid crystalline phase at a certain temperature and pressure (the inversion point) to another phase of the same chemical composition with a different crystal structure." According to McCrone, polymorphs are "different in crystal structure but identical in the liquid or vapor states." Materials with two polymorphs are called dimorphic, with three polymorphs, trimorphic, etc. Examples Many compounds exhibit polymorphism. It has been claimed that "every compound has different polymorphic forms, and that, in general, the n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |