|
Plasminogen Activation System
Plasmin is an important enzyme () present in blood that degrades many blood plasma proteins, including fibrin clots. The degradation of fibrin is termed fibrinolysis. In humans, the plasmin protein (in the zymogen form of plasminogen) is encoded by the ''PLG'' gene. Function Plasmin is a serine protease that acts to dissolve fibrin blood clots. Apart from fibrinolysis, plasmin proteolysis, proteolyses proteins in various other systems: It activates collagenases, some mediators of the complement system, and weakens the wall of the Graafian follicle, leading to ovulation. Plasmin is also integrally involved in inflammation. It cleaves fibrin Fibrin (also called Factor Ia) is a fibrous, non-globular protein involved in the clotting of blood. It is formed by the action of the protease thrombin on fibrinogen, which causes it to polymerize. The polymerized fibrin, together with platele ..., fibronectin, thrombospondin, laminin, and von Willebrand factor. Plasmin, like trypsi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the reaction ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Von Willebrand Factor
Von Willebrand factor (VWF) () is a blood glycoprotein involved in hemostasis, specifically, platelet adhesion. It is deficient and/or defective in von Willebrand disease and is involved in many other diseases, including thrombotic thrombocytopenic purpura, Heyde's syndrome, and possibly hemolytic–uremic syndrome. Increased plasma levels in many cardiovascular, neoplastic, metabolic (e.g. diabetes), and connective tissue diseases are presumed to arise from adverse changes to the endothelium, and may predict an increased risk of thrombosis. Biochemistry Synthesis VWF is a large multimeric glycoprotein present in blood plasma and produced constitutively as ultra-large VWF in endothelium (in the Weibel–Palade bodies), megakaryocytes (α-granules of platelets), and subendothelial connective tissue. Structure The basic VWF monomer is a 2050-amino acid protein. Every monomer contains a number of specific domains with a specific function; elements of note are: * the D'/D3 do ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha 2-antiplasmin
Alpha 2-antiplasmin (or α2-antiplasmin or plasmin inhibitor) is a serine protease inhibitor (serpin) responsible for inactivating plasmin. Plasmin is an important enzyme that participates in fibrinolysis and degradation of various other proteins. This protein is encoded by the SERPINF2 gene. Role in disease Very few cases (<20) of A2AP deficiency have been described. As plasmin degrades blood clots, impaired inhibition of plasmin leads to a bleeding tendency, which was severe in the cases reported. In liver , there is decreased production of alpha 2-antiplasmin, leading to decreased inactivation of and an increase in |
Alpha-2-Macroglobulin
α2-Macroglobulin (α2M), or alpha-2-macroglobulin, is a large (720 KDa) plasma protein found in the blood. It is mainly produced by the liver, and also locally synthesized by macrophages, fibroblasts, and adrenocortical cells. In humans it is encoded by the ''A2M'' gene. α2-Macroglobulin acts as an antiprotease and is able to inactivate an enormous variety of proteinases. It functions as an inhibitor of fibrinolysis by inhibiting plasmin and kallikrein. It functions as an inhibitor of coagulation by inhibiting thrombin. α2-macroglobulin may act as a carrier protein because it also binds to numerous growth factors and cytokines, such as platelet-derived growth factor, basic fibroblast growth factor, TGF-β, insulin, and IL-1β. No specific deficiency with associated disease has been recognized, and no disease state is attributed to low concentrations of α2-macroglobulin. The concentration of α2-macroglobulin rises 10-fold or more in the nephrotic syndrome when other lower mo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Activation Loop
In molecular biology, an intrinsically disordered protein (IDP) is a protein that lacks a fixed or ordered three-dimensional structure, typically in the absence of its macromolecular interaction partners, such as other proteins or RNA. IDPs range from fully unstructured to partially structured and include random coil, molten globule-like aggregates, or flexible linkers in large multi-domain proteins. They are sometimes considered as a separate class of proteins along with globular, fibrous and membrane proteins. IDPs are a very large and functionally important class of proteins and their discovery has disproved the idea that three-dimensional structures of proteins must be fixed to accomplish their biological functions. For example, IDPs have been identified to participate in weak multivalent interactions that are highly cooperative and dynamic, lending them importance in DNA regulation and in cell signaling. Many IDPs can also adopt a fixed three-dimensional structure after bi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
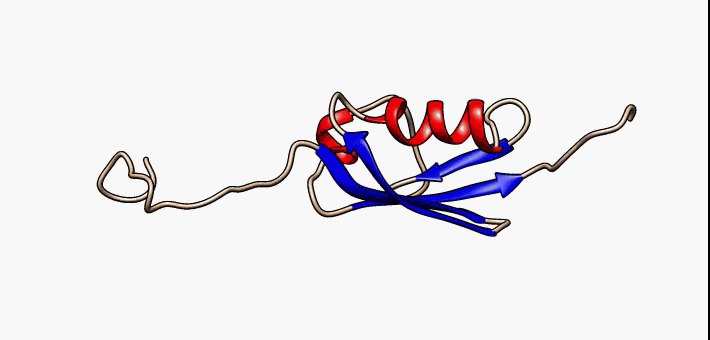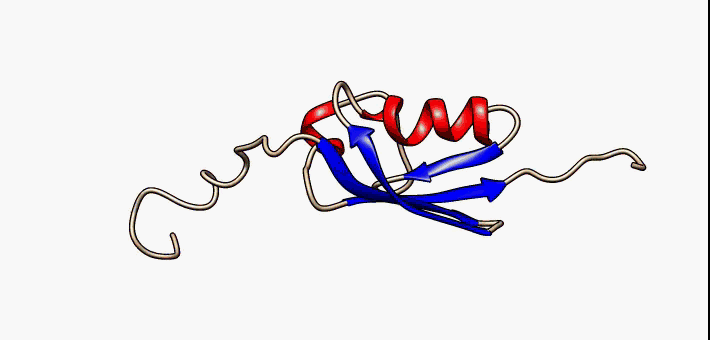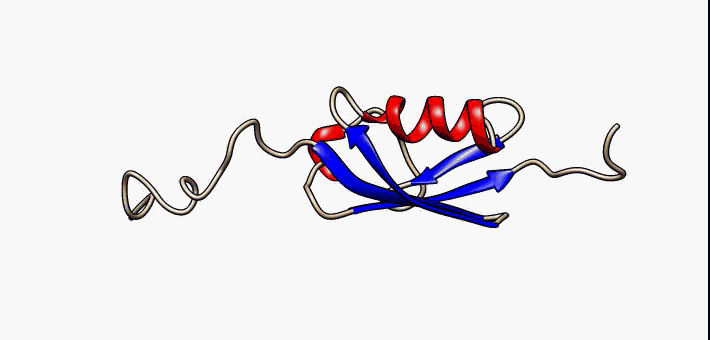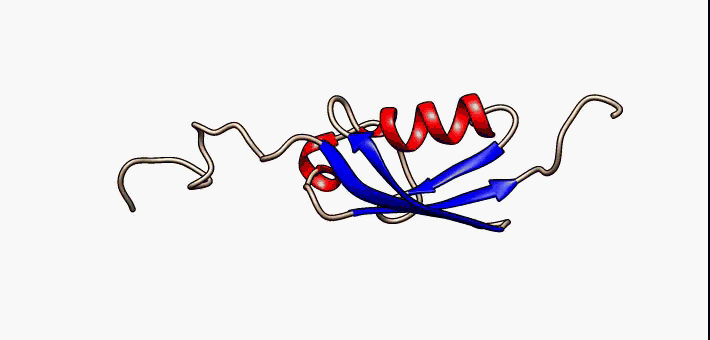
Kringle Domain
Kringle Domains are autonomous protein domains that fold into large loops stabilized by 3 disulfide linkages. These are important in protein–protein interactions with blood coagulation factors. Their name refers to the Kringle, a Scandinavian pastry which they somewhat resemble. Kringle domains have been found in plasminogen, hepatocyte growth factors, prothrombin, and apolipoprotein(a). Kringles are found throughout the blood clotting and fibrinolytic proteins. Kringle domains are believed to play a role in binding mediators (e.g., membranes, other proteins or phospholipids), and in the regulation of proteolytic activity. Kringle domains are characterised by a triple loop, 3-disulfide bridge structure, whose conformation is defined by a number of hydrogen bonds and small pieces of anti-parallel beta-sheet. They are found in a varying number of copies in some plasma proteins including prothrombin and urokinase-type plasminogen activator, which are serine proteases belonging ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PAN Domain
PAN protein domain, domains have significant functional versatility fulfilling diverse biology, biological roles by mediating protein-protein interaction, protein-protein and protein-carbohydrate interactions. These domains contain a Hairpin turn, hair-pin loop like structure, similar to that found in Inhibitor cystine knot, knottins but with a different pattern of disulfide bonds. It has been shown that the N-terminal domains of members of the plasminogen/hepatocyte growth factor family, the apple domains of the blood plasma, plasma prekallikrein/Factor XI, coagulation factor XI family, and domains of various nematode proteins belong to the same module superfamily, the PAN module. The PAN domain contains a conserved sequence, conserved core of three disulfide bridges. In some members of the family there is an additional fourth disulfide bridge that links the N-terminus, N- and C-terminus, C-termini of the domain. The apple domain, as well as other examples of the PAN domain, cons ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Angiostatin
Angiostatin is a naturally occurring protein found in several animal species, including humans. It is an endogenous angiogenesis inhibitor (i.e., it blocks the growth of new blood vessels). Clinical trials have been undertaken for its use in anticancer therapy. Structure Angiostatin is a 38 kDa fragment of a larger protein, plasmin (itself a fragment of plasminogen) enclosing three to five contiguous kringle modules. Each module contains two small beta sheets and three disulfide bonds. There are four different structural variants to angiostatin differing in the combination of kringle domains: K1-3, K1-4, K1-5, K1-4 with a fragment of K-5. Each kringle domain contributes a different element of inhibition to the cytokine. Recent studies through recombinant angiostatin have shown however that K1-3 is pivotal is the inhibitory nature of angiostatin. K1-3 form the “triangular bowl-like structure” of angiostatin. This structure is stabilized by interactions between inter-kringle pep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Urokinase Receptor
The Urokinase receptor, also known as urokinase plasminogen activator surface receptor (uPAR) or CD87 (Cluster of Differentiation 87), is a protein encoded in humans by the PLAUR gene. It is a multidomain glycoprotein tethered to the cell membrane with a (GPI) anchor. uPAR was originally identified as a saturable binding site for urokinase (also known as uPA) on the cell surface. Molecular characteristics uPAR consists of three tandem LU domains, which are protein domains of the three-finger protein family. The structure of uPAR has been solved by X-ray crystallography in complex with a peptide antagonist and with its native ligand, urokinase. All three three-finger domains are necessary for high affinity binding of the primary ligand, urokinase. In addition, uPAR also interacts with several other proteins, including vitronectin, the uPAR associated protein (uPARAP) and the integrin family of membrane proteins. It has been possible to express uPAR recombinantly in CHO-cells ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Factor XII
Coagulation factor XII, also known as Hageman factor, is a plasma protein. It is the zymogen form of factor XIIa, an enzyme () of the serine protease (or serine endopeptidase) class. In humans, factor XII is encoded by the ''F12'' gene. Structure Human Factor XII is 596 amino acids long and consists of two chains, the heavy chain (353 residues) and light chain (243 residues) held together by a disulfide bond. It is 80,000 daltons. Its heavy chain contains two fibronectin-type domains (type I and II), two epidermal growth factor-like domains, a kringle domain, and a proline-rich region, and its light chain contains the protease domain. The structure of the FnI-EGF-like tandem domain of coagulation factor XII has been solved by X-ray crystallography. Crystal structures of the FXII light chain has also been determined unbound (β-FXII) and bound (β-FXIIa) to inhibitors. Factor XII (FXII, Hageman factor) is a plasma glycoprotein of approximately 90 kDa molecular weight is part ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kallikrein
Kallikreins are a subgroup of serine proteases, enzymes capable of cleaving peptide bonds in proteins. In humans, plasma kallikrein (encoded by ''KLKB1 gene'') has no known paralogue, while tissue kallikrein-related peptidases (''KLKs'') encode a family of fifteen closely related serine proteases. These genes are localised to chromosome 19q13, forming the largest contiguous cluster of proteases within the human genome. Kallikreins are responsible for the coordination of various physiological functions including blood pressure, semen liquefaction and skin desquamation. Occurrence In 1934, Eugen Werle reported finding a substance in the pancreas of humans and various animals in such large amounts that the pancreas could be taken for its site of origin. He named it kallikrein, by derivation from the Greek word for pancreas. Since then, similar enzymes have been found in the biological fluids of humans and other mammals, as well as in some snake venoms. Venom The caterpillar known ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Urokinase
Urokinase, also known as urokinase-type plasminogen activator (uPA), is a serine protease present in humans and other animals. The human urokinase protein was discovered, but not named, by McFarlane and Pilling in 1947. Urokinase was originally isolated from human urine, and it is also present in the blood and in the extracellular matrix of many tissues. The primary physiological substrate of this enzyme is plasminogen, which is an inactive form (zymogen) of the serine protease plasmin. Activation of plasmin triggers a proteolytic cascade that, depending on the physiological environment, participates in thrombolysis or extracellular matrix degradation. This cascade had been involved in vascular diseases and cancer progression. Urokinase is encoded in humans by the ''PLAU'' gene, which stands for "plasminogen activator, urokinase". The same symbol represents the gene in other animal species. Function The ''PLAU'' gene encodes a serine protease () involved in degradation of the e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |