|
Phosphoribosylamine
Phosphoribosylamine (PRA) is a biochemical intermediate in the formation of purine nucleotides via inosine-5-monophosphate, and hence is a building block for DNA and RNA. The vitamins thiamine and cobalamin also contain fragments derived from PRA. : It is the product of the enzyme amidophosphoribosyltransferase which attaches ammonia from glutamine to phosphoribosyl pyrophosphate (PRPP) at its anomeric carbon: : + → + + PPi The biosynthesis pathway next combines PRA with glycine in a process driven by ATP giving glycineamide ribonucleotide (GAR). The enzyme phosphoribosylamine—glycine ligase catalyses the reaction forming an amide bond: : + + ATP → + ADP + Pi See also * 5-Aminoimidazole ribotide 5′-Phosphoribosyl-5-aminoimidazole (or aminoimidazole ribotide, AIR) is a biochemical intermediate in the formation of purine nucleotides via inosine-5-monophosphate, and hence is a building block for DNA and RNA. The vitamins thiamine and coba ... * Purine met ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Purine Metabolism
Purine metabolism refers to the metabolic pathways to synthesize and break down purines that are present in many organisms. Biosynthesis Purines are biologically synthesized as nucleotides and in particular as ribotides, i.e. bases attached to ribose 5-phosphate. Both adenine and guanine are derived from the nucleotide inosine monophosphate (IMP), which is the first compound in the pathway to have a completely formed purine ring system. IMP Inosine monophosphate is synthesized on a pre-existing ribose-phosphate through a complex pathway (as shown in the figure on the right). The source of the carbon and nitrogen atoms of the purine ring, 5 and 4 respectively, come from multiple sources. The amino acid glycine contributes all its carbon (2) and nitrogen (1) atoms, with additional nitrogen atoms from glutamine (2) and aspartic acid (1), and additional carbon atoms from formyl groups (2), which are transferred from the coenzyme tetrahydrofolate as 10-formyltetrahydrofolate, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-Aminoimidazole Ribotide
5′-Phosphoribosyl-5-aminoimidazole (or aminoimidazole ribotide, AIR) is a biochemical intermediate in the formation of purine nucleotides via inosine-5-monophosphate, and hence is a building block for DNA and RNA. The vitamins thiamine and cobalamin also contain fragments derived from AIR. It is an intermediate in the adenine pathway and is synthesized from 5′-phosphoribosylformylglycinamidine by AIR synthetase. Chemistry 5-aminoimidazole derivatives were considered unstable and therefore difficult to synthesize. The first non-enzymatic synthesis of 5-aminoimidazole ribotide (AIR) was only published in 1988 and general methodology for other examples was developed in the 1990s. Biosynthesis The furanose (5-carbon) sugar in AIR comes from the pentose phosphate pathway, which converts glucose (as its 6-phosphate derivative) into ribose 5-phosphate (R5P). The subsequent reactions which attach the aminoimidazole portion of the molecule begin when R5P is activated as its pyrop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amidophosphoribosyltransferase
Amidophosphoribosyltransferase (ATase), also known as glutamine phosphoribosylpyrophosphate amidotransferase (GPAT), is an enzyme responsible for catalyzing the conversion of Phosphoribosylpyrophosphate, 5-phosphoribosyl-1-pyrophosphate (PRPP) into Phosphoribosylamine, 5-phosphoribosyl-1-amine (PRA), using the amine group from a glutamine side-chain. This is the committing step in de novo purine synthesis. In humans it is encoded by the ''PPAT'' (phosphoribosyl pyrophosphate amidotransferase) gene. ATase is a member of the purine/pyrimidine phosphoribosyltransferase family. Structure and function The enzyme consists of two domains: a glutaminase domain that produces ammonia from glutamine by hydrolysis and a phosphoribosyltransferase domain that binds the ammonia to ribose-5-phosphate. Coordination between the two active sites of enzyme give it special complexity. The glutaminase domain is homologous to other N-terminal nucleophile (Ntn) hydrolases such as carbamoyl phosphate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphoribosylamine—glycine Ligase
''Phosphoribosylamine—glycine ligase'', also known as glycinamide ribonucleotide synthetase (GARS), () is an enzyme that catalyzes the chemical reaction :ATP + 5-phospho-D-ribosylamine + glycine \rightleftharpoons ADP + phosphate + which is the second step in purine biosynthesis. The 3 substrates of this enzyme are ATP, 5-phospho-D-ribosylamine, and glycine, whereas its 3 products are ADP, phosphate, and . This enzyme belongs to the family of ligases, specifically those forming generic carbon-nitrogen bonds. In bacteria, GARS is a monofunctional enzyme (encoded by the purD gene). The purD genes often contain PurD RNA motif in their 5' UTR. In yeast, GARS is part of a bifunctional enzyme (encoded by the ADE5/7 gene) in conjunction with phosphoribosylformylglycinamidine cyclo-ligase (AIRS). In higher eukaryotes, including humans, GARS is part of a trifunctional enzyme in conjunction with AIRS and with phosphoribosylglycinamide formyltransferase (GART), forming GARS-AIR ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycineamide Ribonucleotide
Glycineamide ribonucleotide (or GAR) is a biochemical intermediate in the formation of purine nucleotides via inosine-5-monophosphate, and hence is a building block for DNA and RNA. The vitamins thiamine and cobalamin also contain fragments derived from GAR. : GAR is the product of the enzyme phosphoribosylamine—glycine ligase acting on phosphoribosylamine (PRA) to combine it with glycine in a process driven by ATP. The reaction, forms an amide bond: : + + ATP → + ADP + Pi The biosynthesis pathway next adds a formyl group from 10-formyltetrahydrofolate to GAR, catalysed by phosphoribosylglycinamide formyltransferase in reaction and producing formylglycinamide ribotide (FGAR): :GAR + 10-formyltetrahydrofolate → FGAR + tetrahydrofolate See also * 5-Aminoimidazole ribotide * Purine metabolism Purine metabolism refers to the metabolic pathways to synthesize and break down purines that are present in many organisms. Biosynthesis Purines are biologically synthesized ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphoribosyl Pyrophosphate
Phosphoribosyl pyrophosphate (PRPP) is a pentose phosphate. It is a biochemical intermediate in the formation of purine nucleotides via inosine-5-monophosphate, as well as in pyrimidine nucleotide formation. Hence it is a building block for DNA and RNA. The vitamins thiamine and cobalamin, and the amino acid tryptophan also contain fragments derived from PRPP. It is formed from ribose 5-phosphate (R5P) by the enzyme ribose-phosphate diphosphokinase: : It plays a role in transferring phospho-ribose groups in several reactions, some of which are salvage pathways: In '' de novo'' generation of purines, the enzyme amidophosphoribosyltransferase acts upon PRPP to create phosphoribosylamine. The histidine biosynthesis pathway involves the reaction between PRPP and ATP, which activates the latter to ring cleavage. Carbon atoms from ribose in PRPP form the linear chain and part of the imidazole ring in histidine. The same is true for the biosynthesis of tryptophan, with the first ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphoribosyl Pyrophosphate
Phosphoribosyl pyrophosphate (PRPP) is a pentose phosphate. It is a biochemical intermediate in the formation of purine nucleotides via inosine-5-monophosphate, as well as in pyrimidine nucleotide formation. Hence it is a building block for DNA and RNA. The vitamins thiamine and cobalamin, and the amino acid tryptophan also contain fragments derived from PRPP. It is formed from ribose 5-phosphate (R5P) by the enzyme ribose-phosphate diphosphokinase: : It plays a role in transferring phospho-ribose groups in several reactions, some of which are salvage pathways: In '' de novo'' generation of purines, the enzyme amidophosphoribosyltransferase acts upon PRPP to create phosphoribosylamine. The histidine biosynthesis pathway involves the reaction between PRPP and ATP, which activates the latter to ring cleavage. Carbon atoms from ribose in PRPP form the linear chain and part of the imidazole ring in histidine. The same is true for the biosynthesis of tryptophan, with the first ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
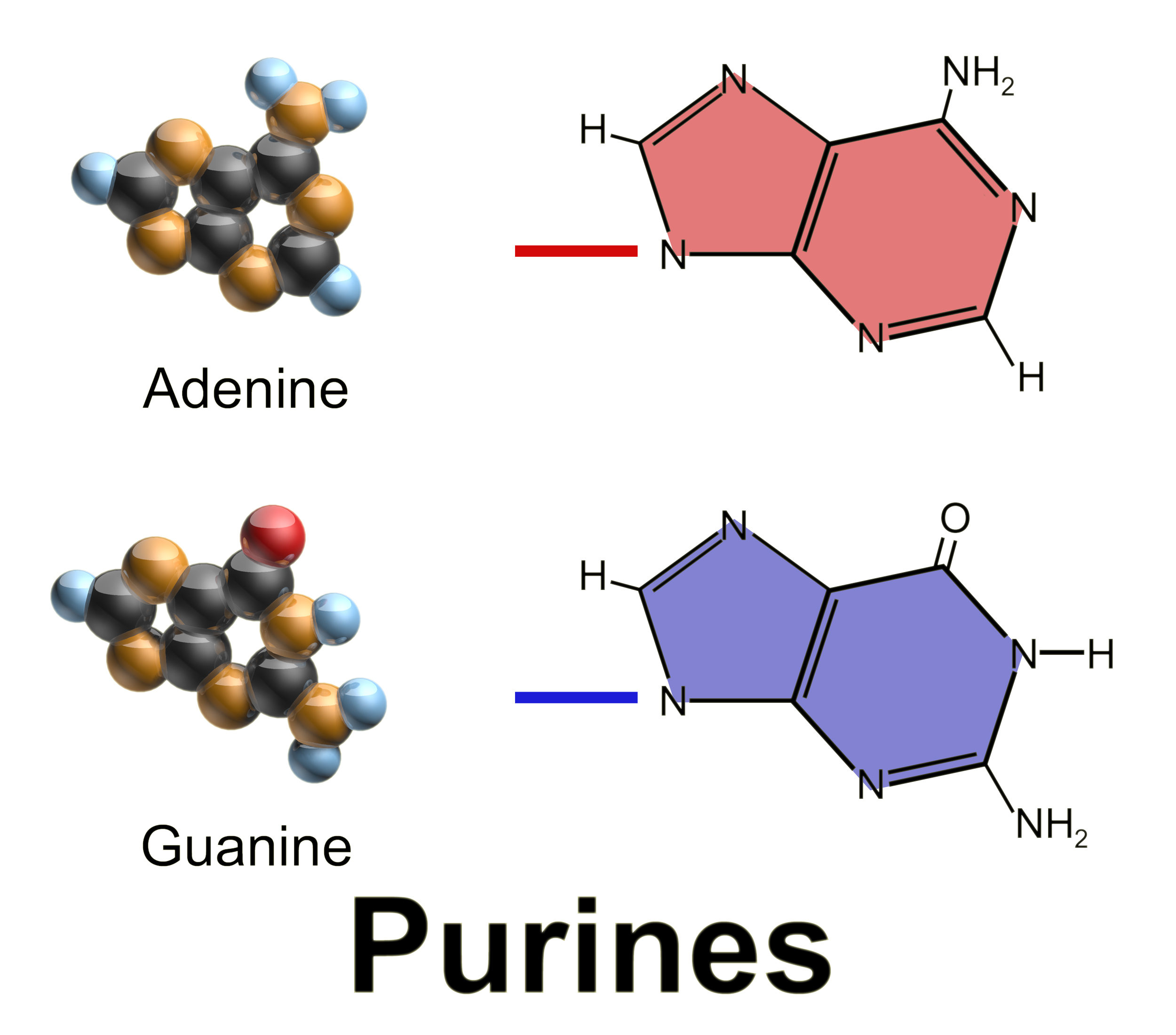
Purine
Purine is a heterocyclic aromatic organic compound that consists of two rings ( pyrimidine and imidazole) fused together. It is water-soluble. Purine also gives its name to the wider class of molecules, purines, which include substituted purines and their tautomers. They are the most widely occurring nitrogen-containing heterocycles in nature. Dietary sources Purines are found in high concentration in meat and meat products, especially internal organs such as liver and kidney. In general, plant-based diets are low in purines. High-purine plants and algae include some legumes (lentils and black eye peas) and spirulina. Examples of high-purine sources include: sweetbreads, anchovies, sardines, liver, beef kidneys, brains, meat extracts (e.g., Oxo, Bovril), herring, mackerel, scallops, game meats, yeast (beer, yeast extract, nutritional yeast) and gravy. A moderate amount of purine is also contained in red meat, beef, pork, poultry, fish and seafood, asparagus, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycine
Glycine (symbol Gly or G; ) is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid ( carbamic acid is unstable), with the chemical formula NH2‐ CH2‐ COOH. Glycine is one of the proteinogenic amino acids. It is encoded by all the codons starting with GG (GGU, GGC, GGA, GGG). Glycine is integral to the formation of alpha-helices in secondary protein structure due to its compact form. For the same reason, it is the most abundant amino acid in collagen triple-helices. Glycine is also an inhibitory neurotransmitter – interference with its release within the spinal cord (such as during a '' Clostridium tetani'' infection) can cause spastic paralysis due to uninhibited muscle contraction. It is the only achiral proteinogenic amino acid. It can fit into hydrophilic or hydrophobic environments, due to its minimal side chain of only one hydrogen atom. History and etymology Glycine was discovered in 1820 by the French ch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it is part of the main chain of a protein, and an isopeptide bond when it occurs in a side chain, such as in the amino acids asparagine and glutamine. It can be viewed as a derivative of a carboxylic acid () with the hydroxyl group () replaced by an amine group (); or, equivalently, an acyl (alkanoyl) group () joined to an amine group. Common examples of amides are acetamide (), benzamide (), and dimethylformamide (). Amides are qualified as primary, secondary, and tertiary according to whether the amine subgroup has the form , , or , where R and R' are groups other than hydrogen. The core of amides is called the amide group (specifically, carboxamide group). Amides are pervasive in nature and technology. Proteins and important p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adenosine Triphosphate
Adenosine triphosphate (ATP) is an organic compound that provides energy to drive many processes in living cells, such as muscle contraction, nerve impulse propagation, condensate dissolution, and chemical synthesis. Found in all known forms of life, ATP is often referred to as the "molecular unit of currency" of intracellular energy transfer. When consumed in metabolic processes, it converts either to adenosine diphosphate (ADP) or to adenosine monophosphate (AMP). Other processes regenerate ATP. The human body recycles its own body weight equivalent in ATP each day. It is also a precursor to DNA and RNA, and is used as a coenzyme. From the perspective of biochemistry, ATP is classified as a nucleoside triphosphate, which indicates that it consists of three components: a nitrogenous base ( adenine), the sugar ribose, and the triphosphate. Structure ATP consists of an adenine attached by the 9-nitrogen atom to the 1′ carbon atom of a sugar ( ribose), which in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anomeric Carbon
In carbohydrate chemistry, a pair of anomers () is a pair of near-identical stereoisomers that differ at only the anomeric carbon, the carbon that bears the aldehyde or ketone functional group in the sugar's open-chain form. However, in order for anomers to exist, the sugar must be in its cyclic form, since in open-chain form, the anomeric carbon is planar and thus achiral. More formally stated, then, an anomer is an epimer at the hemiacetal/hemiketal carbon in a cyclic saccharide. Anomerization is the process of conversion of one anomer to the other. As is typical for stereoisomeric compounds, different anomers have different physical properties, melting points and specific rotations. Nomenclature Two anomers are designated alpha (α) or beta (β), according to the configurational relationship between the ''anomeric centre'' and the ''anomeric reference atom'', hence they are relative stereodescriptors. The anomeric centre in hemiacetals is the anomeric carbon C-1; in hem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |