|
Peroxy
In chemistry, peroxides are a group of compounds with the structure , where R = any element. The group in a peroxide is called the peroxide group or peroxo group. The nomenclature is somewhat variable. The most common peroxide is hydrogen peroxide (), colloquially known simply as "peroxide". It is marketed as solutions in water at various concentrations. Many organic peroxides are known as well. In addition to hydrogen peroxide, some other major classes of peroxides are: * Peroxy acids, the peroxy derivatives of many familiar acids, examples being peroxymonosulfuric acid and peracetic acid, and their salts, one example of which is potassium peroxydisulfate. * Main group peroxides, compounds with the linkage (E = main group element). * Metal peroxides, examples being barium peroxide (), sodium peroxide () and zinc peroxide Zinc peroxide (ZnO2) appears as a bright yellow powder at room temperature. It was historically used as a surgical antiseptic. More recently zin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peroxy Acid
A peroxy acid (often spelled as one word, peroxyacid, and sometimes called peracid) is an acid which contains an acidic –OOH group. The two main classes are those derived from conventional mineral acids, especially sulfuric acid, and the peroxy derivatives of organic carboxylic acids. They are generally strong oxidizers. Inorganic peroxy acids Peroxymonosulfuric acid (Caro's acid) is probably the most important inorganic peracid, at least in terms of its production scale. It is used for the bleaching of pulp and for the detoxification of cyanide in the mining industry. It is produced by treating sulfuric acid with hydrogen peroxide. Peroxymonophosphoric acid (H3PO5) is prepared similarly. Organic peracids Several organic peroxyacids are commercially useful. They can be prepared in several ways. Most commonly, peracids are generated by treating the corresponding carboxylic acid with hydrogen peroxide: :RCO2H + H2O2 RCO3H + H2O A related reaction involves treatment o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Peroxide
In organic chemistry, organic peroxides are organic compounds containing the peroxide functional group (). If the R′ is hydrogen, the compounds are called hydroperoxides, which are discussed in that article. The O−O bond of peroxides easily breaks, producing free radicals of the form (the dot represents an unpaired electron). Thus, organic peroxides are useful as initiators for some types of polymerisation, such as the epoxy resins used in glass-reinforced plastics. MEKP and benzoyl peroxide are commonly used for this purpose. However, the same property also means that organic peroxides can explosively combust. Organic peroxides, like their inorganic counterparts, are often powerful bleaching agents. Types of organic peroxides Tert-Butyl hydroperoxide Structural Formula V2.svg, ''tert''-Butyl hydroperoxide, a hydroperoxide (formula: ROOH) that is used to epoxide alkenes. Dicumyl peroxide.svg, Dicumyl peroxide, a dialkyl peroxide (formula: ROOR) that is used to initiate p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peroxymonosulfuric Acid
Peroxymonosulfuric acid, , also known as persulfuric acid, peroxysulfuric acid, or Caro's acid. In this acid, the S(VI) center adopts its characteristic tetrahedral geometry; the connectivity is indicated by the formula HO–O–S(O)2–OH. It is one of the strongest oxidants known ( ''E''0 = +2.51 V) and is highly explosive. is sometimes confused with , known as peroxydisulfuric acid. The disulfuric acid, which appears to be more widely used as its alkali metal salts, has the structure HO–S(O)2–O–O–S(O)2–OH. History was first described in 1898 by the German chemist Heinrich Caro, after whom it is named. Synthesis and production The laboratory scale preparation of Caro's acid involves the combination of chlorosulfuric acid and hydrogen peroxide: :: + ⇌ + HCl Published patents include more than one reaction for preparation of Caro's acid, usually as an intermediate for the production of potassium monopersulfate (PMPS), a bleaching and oxidizing agen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peracetic Acid
Peracetic acid (also known as peroxyacetic acid, or PAA) is an organic compound with the formula CH3CO3H. This peroxy acid is a colorless liquid with a characteristic acrid odor reminiscent of acetic acid. It can be highly corrosive. Peracetic acid is a weaker acid than the parent acetic acid, with a p''K''a of 8.2. Production Peracetic acid is produced industrially by the autoxidation of acetaldehyde: :O2 + CH3CHO → CH3CO3H It forms upon treatment of acetic acid with hydrogen peroxide with a strong acid catalyst: :H2O2 + CH3CO2H CH3CO3H + H2O As an alternative, acetyl chloride and acetic anhydride can be used to generate a solution of the acid with lower water content. Peracetic acid is generated ''in situ'' by some laundry detergents. This is achieved by the action of bleach activators, such as tetraacetylethylenediamine and sodium nonanoyloxybenzenesulfonate, upon hydrogen peroxide formed from sodium percarbonate in water. The peracetic acid is a more effective bleachi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Peroxydisulfate
Potassium persulfate is the inorganic compound with the formula K2 S2O8. Also known as potassium peroxydisulfate, it is a white solid that is sparingly soluble in cold water, but dissolves better in warm water. This salt is a powerful oxidant, commonly used to initiate polymerizations. Structure The sodium and potassium salts are very similar. In the potasium salt, the O-O distance is 1.495Å. The individual sulfate groups are tetrahedral, with three short S-O distances near 1.43 and one long S-O bond at 1.65Å. Preparation Potassium persulfate can be prepared by electrolysis of a cold solution potassium bisulfate in sulfuric acid at a high current density.{{cite book, last=Brauer, first=Georg, title=Handbook of Preparative Inorganic Chemistry , volume=1 , edition=2nd , year=1963 , publisher=Academic Press , location=New York , isbn=978-0121266011 , page=392 , url=https://books.google.com/books?id=TLYatwAACAAJ&q=Handbook+of+Preparative+Inorganic+Chemistry : 2 KHSO4 → K2S2O ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%–6% by weight) in water for consumer use, and in higher concentrations for industrial use. Concentrated hydrogen peroxide, or " high-test peroxide", decomposes explosively when heated and has been used as a propellant in rocketry. Hydrogen peroxide is a reactive oxygen species and the simplest peroxide, a compound having an oxygen–oxygen single bond. It decomposes slowly when exposed to light, and rapidly in the presence of organic or reactive compounds. It is typically stored with a stabilizer in a weakly acidic solution in a dark bottle to block light. Hydrogen peroxide is found in biological systems including the human body. Enzymes that use or decompose hydrogen peroxide are classified as peroxidases. Properties The boiling poi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metal Peroxide
Metal peroxides are metal-containing compounds with ionically- or covalently-bonded peroxide () groups. This large family of compounds can be divided into ionic and covalent peroxide. The first class mostly contains the peroxides of the alkali and alkaline earth metals whereas the covalent peroxides are represented by such compounds as hydrogen peroxide and peroxymonosulfuric acid (H2SO5). In contrast to the purely ionic character of alkali metal peroxides, peroxides of transition metals have a more covalent character. Bonding in O22− The peroxide ion is composed of two oxygen atoms that are linked by a single bond. The molecular orbital diagram of the peroxide dianion predicts a doubly occupied antibonding π* orbital and a bond order of 1. The bond length is 149 pm, which is larger than in the ground state (triplet oxygen) of the oxygen molecule (3O2, 121 pm). This translates into the smaller force constant of the bond (2.8 N/cm vs. 11.4 N/cm for 3O2) and the lower ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peroxide Group V
In chemistry, peroxides are a group of compounds with the structure , where R = any element. The group in a peroxide is called the peroxide group or peroxo group. The nomenclature is somewhat variable. The most common peroxide is hydrogen peroxide (), colloquially known simply as "peroxide". It is marketed as solutions in water at various concentrations. Many organic peroxides are known as well. In addition to hydrogen peroxide, some other major classes of peroxides are: * Peroxy acids, the peroxy derivatives of many familiar acids, examples being peroxymonosulfuric acid and peracetic acid, and their salts, one example of which is potassium peroxydisulfate. * Main group peroxides, compounds with the linkage (E = main group element). * Metal peroxides, examples being barium peroxide (), sodium peroxide () and zinc peroxide (). * Organic peroxide In organic chemistry, organic peroxides are organic compounds containing the peroxide functional group (). If the R′ is hyd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Main Group Peroxides
Main group peroxides are peroxide derivatives of the main group elements. Many compounds of the main group elements form peroxides, and a few are of commercial significance. Examples With thousands of tons/year being produced annually, the peroxydisulfates, , are preeminent members of this class. These salts serve as initiators for polymerization of acrylates and styrene. At one time, peroxyborates were used in detergents. These salts have been largely replaced by peroxycarbonates. Many peroxides are not commercially valuable but are of academic interest. One example is bis(trimethylsilyl) peroxide Bis(trimethylsilyl)peroxide (sometimes abbreviated as BTSP) is an organosilicon compound with the formula ((CH3)3SiO)2. It is a colorless liquid that is soluble in organic solvents so long as they lack acidic groups. The compound represents an a ... (Me3SiOOSiMe3). Phosphorus oxides form a number of peroxides, e.g. "P2O6".{{Greenwood&Earnshaw2nd References Peroxides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
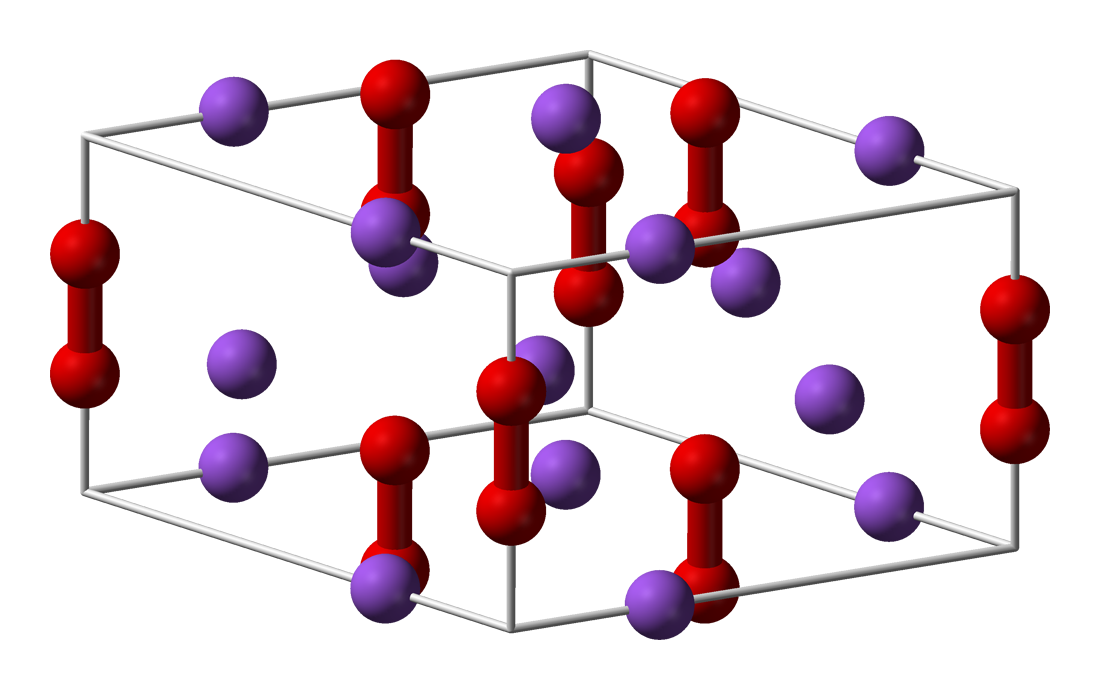
Sodium Peroxide
Sodium peroxide is an inorganic compound with the formula Na2O2. This yellowish solid is the product of sodium ignited in excess oxygen. It is a strong base. This metal peroxide exists in several hydrates and peroxyhydrates including Na2O2·2H2O2·4H2O, Na2O2·2H2O, Na2O2·2H2O2, and Na2O2·8H2O.Harald Jakob, Stefan Leininger, Thomas Lehmann, Sylvia Jacobi, Sven Gutewort. "Peroxo Compounds, Inorganic". ''Ullmann's Encyclopedia of Industrial Chemistry'', 2007, Wiley-VCH, Weinheim. . The octahydrate, which is simple to prepare, is white, in contrast to the anhydrous material. Properties Sodium peroxide crystallizes with hexagonal symmetry. Upon heating, the hexagonal form undergoes a transition into a phase of unknown symmetry at 512 °C. With further heating above the 657 °C boiling point, the compound decomposes to Na2O, releasing O2.Lewis, R. J. ''Sax's Dangerous Properties of Industrial Materials, 10th ed.'', John Wiley & Sons, Inc.: 2000. : 2 Na2O2 → 2 Na2O ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a Chemical reaction, reaction with other Chemical substance, substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both Basic research, basic and Applied science, applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth (botany), the formation of igneous rocks (geology), how atmospheric ozone is formed and how environmental pollutants are degraded (ecology), the properties ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Main Group Element
In chemistry and atomic physics, the main group is the group of elements (sometimes called the representative elements) whose lightest members are represented by helium, lithium, beryllium, boron, carbon, nitrogen, oxygen, and fluorine as arranged in the periodic table of the elements. The main group includes the elements (except hydrogen, which is sometimes not included) in groups 1 and 2 (s-block), and groups 13 to 18 (p-block). The s-block elements are primarily characterised by one main oxidation state, and the p-block elements, when they have multiple oxidation states, often have common oxidation states separated by two units. Main-group elements (with some of the lighter transition metals) are the most abundant elements on Earth, in the Solar System, and in the universe. Group 12 elements are often considered to be transition metals; however, zinc (Zn), cadmium (Cd), and mercury (Hg) share some properties of both groups, and some scientists believe they should be included i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |