|
Pyrimidine Dimer
Pyrimidine dimers are molecular lesions formed from thymine or cytosine bases in DNA via photochemical reactions, commonly associated with direct DNA damage. Ultraviolet light (UV; particularly UVB) induces the formation of covalent linkages between consecutive bases along the nucleotide chain in the vicinity of their carbon–carbon double bonds. The dimerization reaction can also occur among pyrimidine bases in dsRNA (double-stranded RNA)—uracil or cytosine. Two common UV products are cyclobutane pyrimidine dimers (CPDs) and 6–4 photoproducts. These premutagenic lesions alter the structure and possibly the base-pairing. Up to 50–100 such reactions per second might occur in a skin cell during exposure to sunlight, but are usually corrected within seconds by photolyase reactivation or nucleotide excision repair. Uncorrected lesions can inhibit polymerases, cause misreading during transcription or replication, or lead to arrest of replication. It causes sunburn and it trig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DNA Replication
In molecular biology, DNA replication is the biological process of producing two identical replicas of DNA from one original DNA molecule. DNA replication occurs in all living organisms acting as the most essential part for biological inheritance. This is essential for cell division during growth and repair of damaged tissues, while it also ensures that each of the new cells receives its own copy of the DNA. The cell possesses the distinctive property of division, which makes replication of DNA essential. DNA is made up of a double helix of two complementary strands. The double helix describes the appearance of a double-stranded DNA which is thus composed of two linear strands that run opposite to each other and twist together to form. During replication, these strands are separated. Each strand of the original DNA molecule then serves as a template for the production of its counterpart, a process referred to as semiconservative replication. As a result of semi-conservativ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Placentalia
Placental mammals ( infraclass Placentalia ) are one of the three extant subdivisions of the class Mammalia, the other two being Monotremata and Marsupialia. Placentalia contains the vast majority of extant mammals, which are partly distinguished from monotremes and marsupials in that the fetus is carried in the uterus of its mother to a relatively late stage of development. The name is something of a misnomer considering that marsupials also nourish their fetuses via a placenta, though for a relatively briefer period, giving birth to less developed young which are then nurtured for a period inside the mother's pouch. Anatomical features Placental mammals are anatomically distinguished from other mammals by: * a sufficiently wide opening at the bottom of the pelvis to allow the birth of a large baby relative to the size of the mother. * the absence of epipubic bones extending forward from the pelvis, which are found in all other mammals. (Their function in non-placental mammal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleic Acid Structure
Nucleic acid structure refers to the structure of nucleic acids such as DNA and RNA. Chemically speaking, DNA and RNA are very similar. Nucleic acid structure is often divided into four different levels: primary, secondary, tertiary, and quaternary. Primary structure Primary structure consists of a linear sequence of nucleotides that are linked together by phosphodiester bond. It is this linear sequence of nucleotides that make up the primary structure of DNA or RNA. Nucleotides consist of 3 components: # Nitrogenous base ## Adenine ## Guanine ## Cytosine ## Thymine (present in DNA only) ## Uracil (present in RNA only) # 5-carbon sugar which is called deoxyribose (found in DNA) and ribose (found in RNA). # One or more phosphate groups. The nitrogen bases adenine and guanine are purine in structure and form a glycosidic bond between their 9 nitrogen and the 1' -OH group of the deoxyribose. Cytosine, thymine, and uracil are pyrimidines, hence the glycosidic bonds form betwe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SOS Response
The SOS response is a global response to DNA damage in which the cell cycle is arrested and DNA repair and mutagenesis is induced. The system involves the RecA protein ( Rad51 in eukaryotes). The RecA protein, stimulated by single-stranded DNA, is involved in the inactivation of the repressor ( LexA) of SOS response genes thereby inducing the response. It is an error-prone repair system that contributes significantly to DNA changes observed in a wide range of species. Discovery The SOS response was discovered and named by Miroslav Radman in 1975. Mechanism During normal growth, the SOS genes are negatively regulated by LexA repressor protein dimers. Under normal conditions, LexA binds to a 20-bp consensus sequence (the SOS box) in the operator region for those genes. Some of these SOS genes are expressed at certain levels even in the repressed state, according to the affinity of LexA for their SOS box. Activation of the SOS genes occurs after DNA damage by the accumulat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indirect DNA Damage
Indirect, the opposite of direct, may refer to: *Indirect approach, a battle strategy * Indirect DNA damage, caused by UV-photons * Indirect agonist or indirect-acting agonist, a substance that enhances the release or action of an endogenous neurotransmitter *Indirect speech, a form of speech *Indirect costs, costs that are not directly accountable to a particular function or product * Indirect self-reference, describes an object referring to itself indirectly * Indirect effect, a principle of European Community Law * Indirect finance, where borrowers borrow funds from the financial market through indirect means *Indirection In computer programming, indirection (also called dereferencing) is the ability to reference something using a name, reference, or container instead of the value itself. The most common form of indirection is the act of manipulating a value throug ..., the ability to reference something in computer programming * Indirect transmission, infections passing from on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photoprotection
Photoprotection is the biochemical process that helps organisms cope with molecular damage caused by sunlight. Plants and other oxygenic phototrophs have developed a suite of photoprotective mechanisms to prevent photoinhibition and oxidative stress caused by excess or fluctuating light conditions. Humans and other animals have also developed photoprotective mechanisms to avoid UV photodamage to the skin, prevent DNA damage, and minimize the downstream effects of oxidative stress. In photosynthetic organisms In organisms that perform oxygenic photosynthesis, excess light may lead to photoinhibition, or photoinactivation of the reaction centers, a process that does not necessarily involve chemical damage. When photosynthetic antenna pigments such as chlorophyll are excited by light absorption, unproductive reactions may occur by charge transfer to molecules with unpaired electrons. Because oxygenic phototrophs generate O2 as a byproduct from the photocatalyzed splittin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
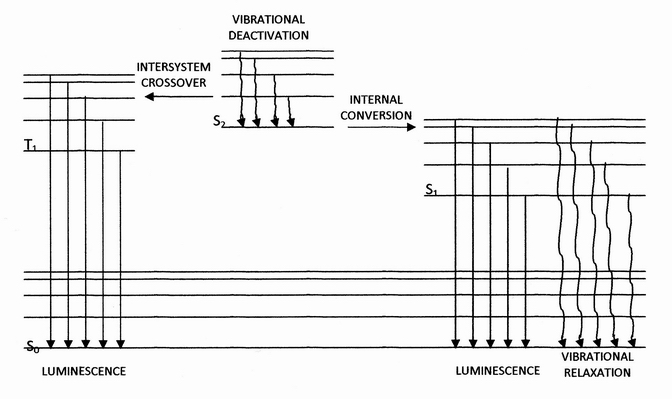
Internal Conversion (chemistry)
Internal conversion is a transition from a higher to a lower electronic state in a molecule or atom.A general and quantitative discussion of intramolecular radiationless transitions is the subject of an article by M. Bixon and J. Jortner (''J. Chem. Phys.'', 48 (2) 715-726 (1968)). It is sometimes called "radiationless de-excitation", because no photons are emitted. It differs from intersystem crossing in that, while both are radiationless methods of de-excitation, the molecular spin state for internal conversion remains the same, whereas it changes for intersystem crossing. The energy of the electronically excited state is given off to vibrational modes of the molecule. The excitation energy is transformed into heat. Examples A classic example of this process is the quinine sulfate fluorescence, which can be quenched by the use of various halide salts. The excited molecule can de-excite by increasing the thermal energy of the surrounding solvated ions. Several natural molecules ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers. Isomers do not necessarily share similar chemical or physical properties. Two main forms of isomerism are structural or constitutional isomerism, in which '' bonds'' between the atoms differ; and stereoisomerism or spatial isomerism, in which the bonds are the same but the ''relative positions'' of the atoms differ. Isomeric relationships form a hierarchy. Two chemicals might be the same constitutional isomer, but upon deeper analysis be stereoisomers of each other. Two molecules that are the same stereoisomer as each other might be in different conformational forms or be different isotopologues. The depth of analysis depends on the field of study or the chemical and physical properties of interest. The English word "isomer" () is a bac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dewar Benzene
Dewar benzene (also spelled ''dewarbenzene'') or bicyclo .2.0exa-2,5-diene is a bicyclic isomer of benzene with the molecular formula C6H6. The compound is named after James Dewar who included this structure in a list of possible C6H6 structures in 1869. However, he did not propose it as the structure of benzene, and in fact he supported the correct structure previously proposed by August Kekulé in 1865. Structure and properties Unlike benzene, Dewar benzene is not flat because the carbons where the rings join are bonded to four atoms rather than three. These carbons tend toward tetrahedral geometry, and the two cyclobutene rings make an angle where they are '' cis''- fused to each other. The compound has nevertheless considerable strain energy and reverts to benzene with a chemical half-life of two days. This thermal conversion is relatively slow because it is symmetry forbidden based on orbital symmetry arguments. Synthesis The compound itself was first synthesized in 1962 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrimidone
Pyrimidone is the name given to either of two heterocyclic compounds with the formula C4H4N2O: 2-pyrimidone and 4-pyrimidone. The compounds can also be called ''2-hydroxypyrimidine'' or ''4-hydroxypyrimidine'' respectively, based on a substituted pyrimidine, or 1,3-diazine, ring. Derivatives Derivatives of pyrimidone are the basis of many other biological molecules, including: * Nucleobases, such as cytosine * Barbiturates, such as metharbital File:Cytosine chemical structure.png, Cytosine Cytosine () (symbol C or Cyt) is one of the four nucleobases found in DNA and RNA, along with adenine, guanine, and thymine ( uracil in RNA). It is a pyrimidine derivative, with a heterocyclic aromatic ring and two substituents attached ... File:Metharbital.png, Metharbital *Antiulcer drugs including temelastine, icotidine, donetidine, and lupitidine. {{Heterocyclic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thymine Photodimer
Thymine () (symbol T or Thy) is one of the four nucleobases in the nucleic acid of DNA that are represented by the letters G–C–A–T. The others are adenine, guanine, and cytosine. Thymine is also known as 5-methyluracil, a pyrimidine nucleobase. In RNA, thymine is replaced by the nucleobase uracil. Thymine was first isolated in 1893 by Albrecht Kossel and Albert Neumann from calf thymus glands, hence its name. Derivation As its alternate name (5-methyluracil) suggests, thymine may be derived by methylation of uracil at the 5th carbon. In RNA, thymine is replaced with uracil in most cases. In DNA, thymine (T) binds to adenine (A) via two hydrogen bonds, thereby stabilizing the nucleic acid structures. Thymine combined with deoxyribose creates the nucleoside deoxythymidine, which is synonymous with the term thymidine. Thymidine can be phosphorylated with up to three phosphoric acid groups, producing dTMP (deoxythymidine monophosphate), dTDP, or dTTP (for the di- and tri- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |