|
Pyridinium Chlorochromate
Pyridinium chlorochromate (PCC) is a yellow-orange salt with the formula 5H5NHsup>+ rO3Clsup>−. It is a reagent in organic synthesis used primarily for oxidation of alcohols to form carbonyls. A variety of related compounds are known with similar reactivity. PCC offers the advantage of the selective oxidation of alcohols to aldehydes or ketones, whereas many other reagents are less selective. Structure and preparation PCC consists of a pyridinium cation, 5H5NHsup>+, and a tetrahedral chlorochromate anion, rO3Clsup>−. Related salts are also known, such as 1-butylpyridinium chlorochromate, 5H5N(C4H9)CrO3Cl] and potassium chlorochromate. PCC is commercially available. Discovered by accident, the reagent was originally prepared via addition of pyridine into a cold solution of chromium trioxide in concentrated hydrochloric acid: :C5H5N + HCl + CrO3 → 5H5NHCrO3Cl] In one alternative method, formation of chromyl chloride (CrO2Cl2) fume during the making of the aforementi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetone
Acetone (2-propanone or dimethyl ketone), is an organic compound with the formula . It is the simplest and smallest ketone (). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odour. Acetone is miscible with water and serves as an important organic solvent in its own right, in industry, home, and laboratory. About 6.7 million tonnes were produced worldwide in 2010, mainly for use as a solvent and production of methyl methacrylate (and from that PMMA) as well as bisphenol A.Acetone World Petrochemicals report, January 2010Stylianos Sifniades, Alan B. Levy, "Acetone" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. It is a common building block in |
Tetrahedron (journal)
''Tetrahedron'' is a weekly peer-reviewed scientific journal covering the field of organic chemistry. According to the ''Journal Citation Reports'', ''Tetrahedron'' has a 2020 impact factor of 2.457. ''Tetrahedron'' and Elsevier Elsevier () is a Dutch academic publishing company specializing in scientific, technical, and medical content. Its products include journals such as '' The Lancet'', '' Cell'', the ScienceDirect collection of electronic journals, '' Trends'', ..., its publisher, support an annual symposium. In 2010, complaints were raised over its high subscription cost. Notable papers , the Web of Science lists ten papers from ''Tetrahedron'' that have more than 1000 citations. The four articles that have been cited more than 2000 times are: * – cited 2228 times * – cited 2162 times * – cited 2124 times * – cited 2107 times See also * '' Tetrahedron Letters'' * '' Tetrahedron Computer Methodology'' * '' Polyhedron'' (journal) Ref ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Example Of PCC Oxidation Of Secondary Alcohol
Example may refer to: * '' exempli gratia'' (e.g.), usually read out in English as "for example" * .example, reserved as a domain name that may not be installed as a top-level domain of the Internet ** example.com, example.net, example.org, example.edu, second-level domain names reserved for use in documentation as examples * HMS ''Example'' (P165), an Archer-class patrol and training vessel of the Royal Navy Arts * ''The Example'', a 1634 play by James Shirley * ''The Example'' (comics), a 2009 graphic novel by Tom Taylor and Colin Wilson * Example (musician), the British dance musician Elliot John Gleave (born 1982) * ''Example'' (album), a 1995 album by American rock band For Squirrels See also * * Exemplar (other), a prototype or model which others can use to understand a topic better * Exemplum, medieval collections of short stories to be told in sermons * Eixample The Eixample (; ) is a district of Barcelona between the old city (Ciutat Vella) and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical And Pharmaceutical Bulletin
''Chemical and Pharmaceutical Bulletin'' is a monthly peer reviewed medical journal published by the Pharmaceutical Society of Japan. The journal was established in 1953 as the ''Pharmaceutical Bulletin''. From 1958 to 2011 it was known as the ''Chemical & Pharmaceutical Bulletin'', and as ''Chemical and Pharmaceutical Bulletin'' from 2012 onward. In 2012, the society re-organized its journals, and most material published in the '' Journal of Health Science'' now started to be published in the sister publication '' Biological and Pharmaceutical Bulletin'' and with some being published in ''Chemical and Pharmaceutical Bulletin''. The editor in chief is Yoshiji Takemoto (Kyoto University). Abstracting and indexing ''Chemical and Pharmaceutical Bulletin'' is abstracted and indexed in the following databases: * Aquatic Sciences & Fisheries Abstracts *Biosis *Biotechnology Research Abstracts *Chemical Abstracts Core * Chimica * International Pharmaceutical Abstracts *MEDLINE *Science ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
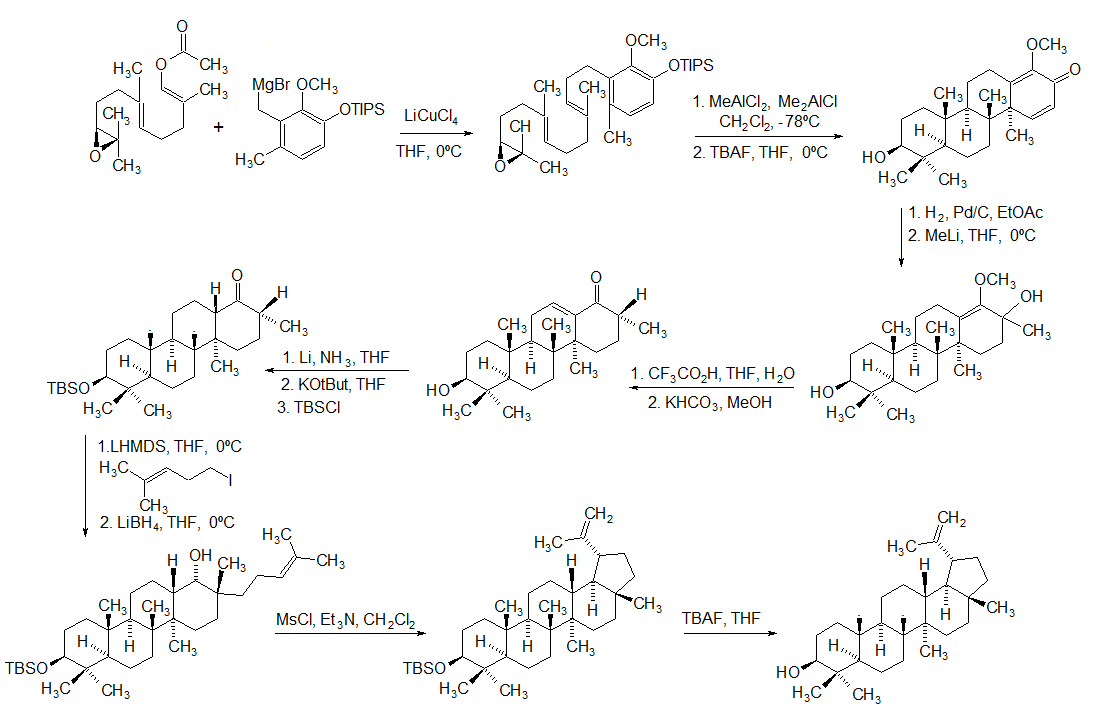
Lupeol
Lupeol is a pharmacologically active pentacyclic triterpenoid. It has several potential medicinal properties, like anticancer and anti-inflammatory activity. Natural occurrences Lupeol is found in a variety of plants, including mango, ''Acacia visco'' and ''Abronia villosa''. It is also found in dandelion coffee. Lupeol is present as a major component in ''Camellia japonica'' leaf. Total synthesis The first total synthesis of lupeol was reported by Gilbert Stork ''et al''. In 2009, Surendra and Corey reported a more efficient and enantioselective total synthesis of lupeol, starting from (''1E,5E'')-8- ''2S'')-3,3-dimethyloxiran-2-yl2,6-dimethylocta-1,5-dienyl acetate by use of a polycyclization. Biosynthesis Lupeol is produced by several organisms from squalene epoxide. Dammarane and baccharane skeletons are formed as intermediates. The reactions are catalyzed by the enzyme lupeol synthase. A recent study on the metabolomics of ''Camellia japonica'' leaf revealed th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triterpene
Triterpenes are a class of chemical compounds composed of three terpene units with the molecular formula C30H48; they may also be thought of as consisting of six isoprene units. Animals, plants and fungi all produce triterpenes, including squalene, the precursor to all steroids. Structures Triterpenes exist in a great variety of structures. Nearly 200 different skeletons have been identified. These skeletons may be broadly divided according to the number of rings present. In general pentacyclic structures (5 rings) tend to dominate. Squalene is biosynthesized through the head-to-head condensation of two farnesyl pyrophosphate units. This coupling converts a pair of C15 components into a C30 product. Squalene serves as precursor for the formation of many triterpenoids, including bacterial hopanoids and eukaryotic sterols. Triterpenoids By definition triterpenoids are triterpenes that possess heteroatoms, usually oxygen. The terms ''triterpene'' and ''triterpenoid'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dichloromethane
Dichloromethane (DCM or methylene chloride, methylene bichloride) is an organochlorine compound with the formula . This colorless, volatile liquid with a chloroform-like, sweet odour is widely used as a solvent. Although it is not miscible with water, it is slightly polar, and miscible with many organic solvents.Rossberg, M. ''et al.'' (2006) "Chlorinated Hydrocarbons" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim. . Occurrence Natural sources of dichloromethane include oceanic sources, macroalgae, wetlands, and volcanoes. However, the majority of dichloromethane in the environment is the result of industrial emissions. Production DCM is produced by treating either chloromethane or methane with chlorine gas at 400–500 °C. At these temperatures, both methane and chloromethane undergo a series of reactions producing progressively more chlorinated products. In this way, an estimated 400,000 tons were produced in the US, Europe, and Japan in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Permanganate
Potassium permanganate is an inorganic compound with the chemical formula KMnO4. It is a purplish-black crystalline salt, that dissolves in water as K+ and , an intensely pink to purple solution. Potassium permanganate is widely used in the chemical industry and laboratories as a strong oxidizing agent, and also as a medication for dermatitis, for cleaning wounds, and general disinfection. It is on the World Health Organization's List of Essential Medicines. In 2000, worldwide production was estimated at 30,000 tonnes. Properties Potassium permanganate is the potassium salt of the tetrahedral transition metal oxo complex permanganate, in which four O2- ligands are bound to a manganese(VII) center. Structure KMnO4 forms orthorhombic crystals with constants: ''a'' = 910.5 pm, ''b'' = 572.0 pm, ''c'' = 742.5 pm. The overall motif is similar to that for barium sulfate, with which it forms solid solutions. In the solid (as in solution), each MnO4− c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxylic Acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion. Examples and nomenclature Carboxylic acids are commonly identified by their trivial names. They at oftentimes have the suffix ''-ic acid''. IUPAC-recommended names also exist; in this system, carboxylic acids have an ''-oic acid'' suffix. For example, butyric acid (C3H7CO2H) is butanoic acid by IUPAC guidelines. For nomenclature of complex molecules containing a carboxylic acid, the carboxyl can be considered position one of the parent chain even if there are other substituents, such as 3-chloropropanoic acid. Alternately, it can be named as a "carboxy" or "carboxylic acid" substituent on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jones' Reagent
The Jones oxidation is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones, respectively. It is named after its discoverer, Sir Ewart Jones. The reaction was an early method for the oxidation of alcohols. Its use has subsided because milder, more selective reagents have been developed, e.g. Collins reagent. Jones reagent is a solution prepared by dissolving chromium trioxide in aqueous sulfuric acid. To effect a Jones oxidation, this acidic mixture is then added to an acetone solution of the substrate. Alternatively, potassium dichromate can be used in place of chromium trioxide. The oxidation is very rapid and quite exothermic. Yields are typically high. The reagent is convenient and cheap. However, Cr(VI) compounds are carcinogenic, which deters the use of this methodology. Stoichiometry and mechanism Jones reagent will convert primary and secondary alcohols to aldehydes and ketones, respectively. Depending on the reaction ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bond C=O). The simplest ketone is acetone (where R and R' is methyl), with the formula . Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids (e.g., testosterone), and the solvent acetone. Nomenclature and etymology The word ''ketone'' is derived from ''Aketon'', an old German word for ''acetone''. According to the rules of IUPAC nomenclature, ketone names are derived by changing the suffix ''-ane'' of the parent alkane to ''-anone''. Typically, the position of the carbonyl group is denoted by a number, but traditional nonsystematic names are still generally used for the most important ketones, for example acetone and benzophenone. These nonsystematic names are considered reta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |