|
Ptaquiloside
Ptaquiloside is a norsesquiterpene glucoside produced by bracken ferns (majorly ''Pteridium aquilinum'') during metabolism. It is identified to be the main carcinogen of the ferns and to be responsible for their biological effects, such as bleeding, haemorrhagic disease and bright blindness in livestock and oesophageal cancer, oesophageal, gastric cancer in humans. Ptaquiloside has unstable chemical structure and acts as a DNA alkylation, alkylating agent under physiological conditions. It was first isolated and characterized by Yamada and co-workers in 1983. The pure form ptaquiloside is a colorless amorphous compound. It is readily soluble in water and fairly soluble in ethyl acetate. Except in the plants, ptaquiloside has been detected in the milk and meat of affected livestock, as well as in the underground water and dry soil around bracken fern vegetation. The prevalence of ptaquiloside in daily sources along with its carcinogenic effects make it an increasing biological hazar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bracken
Bracken (''Pteridium'') is a genus of large, coarse ferns in the family Dennstaedtiaceae. Ferns (Pteridophyta) are vascular plants that have alternating generations, large plants that produce spores and small plants that produce sex cells (eggs and sperm). Brackens are noted for their large, highly divided leaves. They are found on all continents except Antarctica and in all environments except deserts, though their typical habitat is moorland. The genus probably has the widest distribution of any fern in the world. The word ''bracken'' is of Old Norse origin, related to Swedish ''bräken'' and Danish ''bregne'', both meaning fern. In the past, the genus was commonly treated as having only one species, ''Pteridium aquilinum'', but the recent trend is to subdivide it into about ten species. Like other ferns, brackens do not have seeds or fruits, but the immature fronds, known as ''fiddleheads'', are sometimes eaten, although some are thought to be carcinogenic. Description an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pteridium Aquilinum
''Pteridium aquilinum'' (bracken, brake or common bracken), also known as eagle fern, is a species of fern occurring in temperate and subtropical regions in both hemispheres. Originally native to Eurasia and North America, the extreme lightness of its spores has led to it achieving a cosmopolitan distribution. Etymology Common bracken was first described as ''Pteris aquilina'' by Carl Linnaeus, in Volume 2 of his ''Species Plantarum'' in 1753. The origin of the specific epithet derived from the Latin ''aquila'' "eagle". In the reprint of the ''Flora Suecica'' in 1755, Linnaeus explains that the name refers to the image of an eagle seen in the transverse section of the root. In spite of this, the opinion has been forwarded that the name pertains to the shape of the mature fronds appearing akin to an eagle's wing. However, medieval scholars, including Erasmus, thought the pattern of the fibres seen in a transverse section of the stipe resembled a double-headed eagle or oak tree. Ta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Norsesquiterpene
Sesquiterpenes are a class of terpenes that consist of three isoprene units and often have the molecular formula C15H24. Like monoterpenes, sesquiterpenes may be cyclic or contain rings, including many unique combinations. Biochemical modifications such as oxidation or rearrangement produce the related sesquiterpenoids. Sesquiterpenes are found naturally in plants and insects, as semiochemicals, e.g. defensive agents or pheromones. Biosynthesis and examples The reaction of geranyl pyrophosphate with isopentenyl pyrophosphate results in the 15-carbon farnesyl pyrophosphate (FPP), which is an intermediate in the biosynthesis of sesquiterpenes such as farnesene. Cyclic sesquiterpenes are more common than cyclic monoterpenes because of the increased chain length and additional double bond in the sesquiterpene precursors. In addition to common six-membered ring systems such as the ones found in zingiberene and bisacurone, cyclization of one end of the chain to the other end can le ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
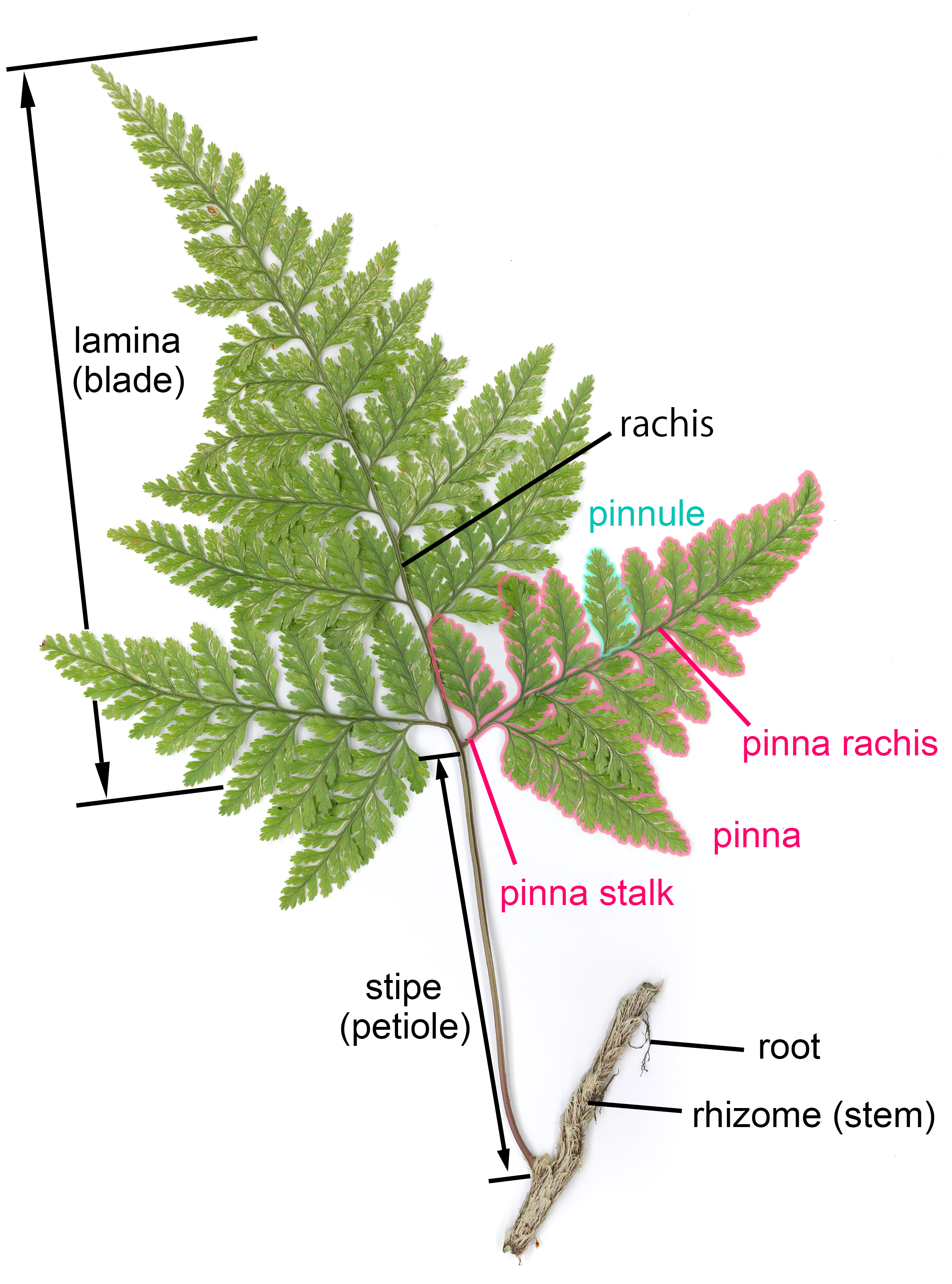
Frond
A frond is a large, divided leaf. In both common usage and botanical nomenclature, the leaves of ferns are referred to as fronds and some botanists restrict the term to this group. Other botanists allow the term frond to also apply to the large leaves of cycads, as well as palms (Arecaceae) and various other flowering plants, such as mimosa or sumac. "Frond" is commonly used to identify a large, compound leaf, but if the term is used botanically to refer to the leaves of ferns and algae it may be applied to smaller and undivided leaves. Fronds have particular terms describing their components. Like all leaves, fronds usually have a stalk connecting them to the main stem. In botany, this leaf stalk is generally called a petiole, but in regard to fronds specifically it is called a stipe, and it supports a flattened blade (which may be called a lamina), and the continuation of the stipe into this portion is called the rachis. The blades may be simple (undivided), pinnatifid ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleotide
Nucleotides are organic molecules consisting of a nucleoside and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both of which are essential biomolecules within all life-forms on Earth. Nucleotides are obtained in the diet and are also synthesized from common nutrients by the liver. Nucleotides are composed of three subunit molecules: a nucleobase, a five-carbon sugar (ribose or deoxyribose), and a phosphate group consisting of one to three phosphates. The four nucleobases in DNA are guanine, adenine, cytosine and thymine; in RNA, uracil is used in place of thymine. Nucleotides also play a central role in metabolism at a fundamental, cellular level. They provide chemical energy—in the form of the nucleoside triphosphates, adenosine triphosphate (ATP), guanosine triphosphate (GTP), cytidine triphosphate (CTP) and uridine triphosphate (UTP)—throughout the cell for the many cellular func ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleoside
Nucleosides are glycosylamines that can be thought of as nucleotides without a phosphate group. A nucleoside consists simply of a nucleobase (also termed a nitrogenous base) and a five-carbon sugar (ribose or 2'-deoxyribose) whereas a nucleotide is composed of a nucleobase, a five-carbon sugar, and one or more phosphate groups. In a nucleoside, the anomeric carbon is linked through a glycosidic bond to the N9 of a purine or the N1 of a pyrimidine. Nucleotides are the molecular building-blocks of DNA and RNA. List of nucleosides and corresponding nucleobases The reason for 2 symbols, shorter and longer, is that the shorter ones are better for contexts where explicit disambiguation is superfluous (because context disambiguates) and the longer ones are for contexts where explicit disambiguation is judged to be needed or wise. For example, when discussing long nucleobase sequences in genomes, the CATG symbol system is much preferable to the Cyt-Ade-Thy-Gua symbol system (see '' N ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha amino acids appear in the genetic code. Amino acids can be classified according to the locations of the core structural functional groups, as Alpha and beta carbon, alpha- , beta- , gamma- or delta- amino acids; other categories relate to Chemical polarity, polarity, ionization, and side chain group type (aliphatic, Open-chain compound, acyclic, aromatic, containing hydroxyl or sulfur, etc.). In the form of proteins, amino acid '' residues'' form the second-largest component (water being the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling life ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are Lewis bases. ''Nucleophilic'' describes the affinity of a nucleophile to bond with positively charged atomic nuclei. Nucleophilicity, sometimes referred to as nucleophile strength, refers to a substance's nucleophilic character and is often used to compare the affinity of atoms. Neutral nucleophilic reactions with solvents such as alcohols and water are named solvolysis. Nucleophiles may take part in nucleophilic substitution, whereby a nucleophile becomes attracted to a full or partial positive charge, and nucleophilic addition. Nucleophilicity is closely related to basicity. History The terms ''nucleophile'' and ''electrophile'' were introduced by Christopher Kelk Ingold in 1933, replacing the terms ''anionoid'' and ''cationoid' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aromatization
Aromatization is a chemical reaction in which an aromatic system is formed from a single nonaromatic precursor. Typically aromatization is achieved by dehydrogenation of existing cyclic compounds, illustrated by the conversion of cyclohexane into benzene. Aromatization includes the formation of heterocyclic systems. : Industrial practice Although not practiced under the name, aromatization is a cornerstone of oil refining. One of the major reforming reactions is the dehydrogenation of naphthenes into aromatics. The process, which is catalyzed by platinum, is exemplified in the conversion methylcyclohexane (a naphthene) into toluene (an aromatic). Dehydrocyclization converts paraffins (acyclic hydrocarbons) into aromatics. A related aromatization process includes dehydroisomerization of methylcyclopentane to benzene: : Biochemical processes : Aromatases are enzymes that aromatize rings within steroids. The specific conversions are testosterone to estradiol and androstenedi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bond C=O). The simplest ketone is acetone (where R and R' is methyl), with the formula . Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids (e.g., testosterone), and the solvent acetone. Nomenclature and etymology The word ''ketone'' is derived from ''Aketon'', an old German word for ''acetone''. According to the rules of IUPAC nomenclature, ketone names are derived by changing the suffix ''-ane'' of the parent alkane to ''-anone''. Typically, the position of the carbonyl group is denoted by a number, but traditional nonsystematic names are still generally used for the most important ketones, for example acetone and benzophenone. These nonsystematic names are considere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carries a partial positive charge, or have an atom that does not have an octet of electrons. Electrophiles mainly interact with nucleophiles through addition and substitution reactions. Frequently seen electrophiles in organic syntheses include cations such as H+ and NO+, polarized neutral molecules such as HCl, alkyl halides, acyl halides, and carbonyl compounds, polarizable neutral molecules such as Cl2 and Br2, oxidizing agents such as organic peracids, chemical species that do not satisfy the octet rule such as carbenes and radicals, and some Lewis acids such as BH3 and DIBAL. Organic chemistry Addition of halogens These occur between alkenes and electrophiles, often halogens as in halogen addition reactions. Common reactions i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopropyl Group
A cyclopropyl group is a chemical structure derived from cyclopropane, and can participate in organic reactions that constitute cycloadditions and rearrangement organic reactions of cyclopropane. The group has an empirical formula of C3H5 and chemical bond A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of ...s from each of the three carbons to both of the other two. Structure and bonding Due to the unfavoured bond angles (60°), cyclopropyl groups are highly strained. Two orbital models were proposed to describe the bonding situation. The Coulson-Moffit model uses bent bonds. The C-C bonds are formed by overlap of two sp-hybrid orbitals. To adapt to the small bond angle, there is some rehybridization resulting in sp~5-hybrids for the ring bonds and sp~2 for the C-H bonds. This model ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |