|
Protomer
In structural biology, a protomer is the structural unit of an oligomeric protein. It is the smallest unit composed of at least two different protein chains that form a larger hetero-oligomer by association of two or more copies of this unit. The term was introduced by Chetverin to make nomenclature in the Na/K-ATPase enzyme unambiguous. This enzyme is composed of two subunits: a large, catalytic α subunit, and a smaller glycoprotein β subunit (plus a proteolipid, called γ-subunit). At the time it was unclear how many of each work together. In addition, when people spoke of a dimer, it was unclear whether they were referring to αβ or to (αβ)2. Chetverin suggested to call αβ a protomer and (αβ)2 a diprotomer. Protomers usually arrange in cyclic symmetry to form closed point group symmetries. In chemistry, a so-called protomer is a molecule which displays tautomerism due to position of a proton. Examples Hemoglobin is a heterotetramer consisting of four subunits ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Capsid
A capsid is the protein shell of a virus, enclosing its genetic material. It consists of several oligomeric (repeating) structural subunits made of protein called protomers. The observable 3-dimensional morphological subunits, which may or may not correspond to individual proteins, are called capsomeres. The proteins making up the capsid are called capsid proteins or viral coat proteins (VCP). The capsid and inner genome is called the nucleocapsid. Capsids are broadly classified according to their structure. The majority of the viruses have capsids with either Helix, helical or icosahedral structure. Some viruses, such as bacteriophages, have developed more complicated structures due to constraints of elasticity and electrostatics. The icosahedral shape, which has 20 equilateral triangular faces, approximates a sphere, while the helical shape resembles the shape of a Spring (device), spring, taking the space of a cylinder but not being a cylinder itself. The capsid faces may ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Structural Biology
Structural biology is a field that is many centuries old which, and as defined by the Journal of Structural Biology, deals with structural analysis of living material (formed, composed of, and/or maintained and refined by living cells) at every level of organization. Early structural biologists throughout the 19th and early 20th centuries were primarily only able to study structures to the limit of the naked eye's visual acuity and through magnifying glasses and light microscopes. In the 20th century, a variety of experimental techniques were developed to examine the 3D structures of biological molecules. The most prominent techniques are X-ray crystallography, nuclear magnetic resonance, and electron microscopy. Through the discovery of X-rays and its applications to protein crystals, structural biology was revolutionized, as now scientists could obtain the three-dimensional structures of biological molecules in atomic detail. Likewise, NMR spectroscopy allowed information about ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a natural science that covers the elements that make up matter to the compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a reaction with other substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both basic and applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth ( botany), the formation of igneous rocks ( geology), how atmospheric ozone is formed and how environmental pollutants are degraded ( ecology), the properties of the soil on the moon ( cosmochemistry), how medications work (pharmacology), and how to collect DNA ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4-aminobenzoic Acid
4-Aminobenzoic acid (also known as ''para''-aminobenzoic acid or PABA because the two functional groups are attached to the benzene ring across from one another in the ''para'' position) is an organic compound with the formula H2NC6H4CO2H. PABA is a white solid, although commercial samples can appear gray. It is slightly soluble in water. It consists of a benzene ring substituted with amino and carboxyl groups. The compound occurs extensively in the natural world. Production and occurrence In industry, PABA is prepared mainly by two routes: * Reduction of 4-nitrobenzoic acid * Hoffman degradation of the monoamide derived from terephthalic acid. Food sources of PABA include liver, brewer's yeast (and unfiltered beer), kidney, molasses, mushrooms, and whole grains. A review on this compound. Biology Biochemistry PABA is an intermediate in the synthesis of folate by bacteria, plants, and fungi. Many bacteria, including those found in the human intestinal tract such a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tyrosine
-Tyrosine or tyrosine (symbol Tyr or Y) or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a non-essential amino acid with a polar side group. The word "tyrosine" is from the Greek ''tyrós'', meaning ''cheese'', as it was first discovered in 1846 by German chemist Justus von Liebig in the protein casein from cheese. It is called tyrosyl when referred to as a functional group or side chain. While tyrosine is generally classified as a hydrophobic amino acid, it is more hydrophilic than phenylalanine. It is encoded by the codons UAC and UAU in messenger RNA. Functions Aside from being a proteinogenic amino acid, tyrosine has a special role by virtue of the phenol functionality. It occurs in proteins that are part of signal transduction processes and functions as a receiver of phosphate groups that are transferred by way of protein kinases. Phosphorylation of the hydroxyl group can change the activity of the targ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aspartate Carbamoyltransferase
Aspartate carbamoyltransferase (also known as aspartate transcarbamoylase or ATCase) catalyzes the first step in the pyrimidine biosynthetic pathway (). In ''E. coli'', the enzyme is a multi- subunit protein complex composed of 12 subunits (300 kDa in total). The composition of the subunits is C6R6, forming 2 trimers of catalytic subunits (34 kDa) and 3 dimers of regulatory subunits (17 kDa). The particular arrangement of catalytic and regulatory subunits in this enzyme affords the complex with strongly allosteric behaviour with respect to its substrates. The enzyme is an archetypal example of allosteric modulation of fine control of metabolic enzyme reactions. ATCase does not follow Michaelis–Menten kinetics. Instead, it lies between its low-activity, low-affinity "tense" and its high-activity, high-affinity "relaxed" states. The binding of substrate to the catalytic subunits results in an equilibrium shift towards the R state, whereas binding of CTP to the regulatory subu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterotetramer
A tetrameric protein is a protein with a quaternary structure of four subunits (tetrameric). Homotetramers have four identical subunits (such as glutathione S-transferase), and heterotetramers are complexes of different subunits. A tetramer can be assembled as dimer of dimers with two homodimer subunits (such as sorbitol dehydrogenase), or two heterodimer subunits (such as hemoglobin). Subunit interactions in tetramers The interactions between subunits forming a tetramer is primarily determined by non covalent interaction. Hydrophobic effects, hydrogen bonds and electrostatic interactions are the primary sources for this binding process between subunits. For homotetrameric proteins such as Sorbitol dehydrogenase (SDH), the structure is believed to have evolved going from a monomeric to a dimeric and finally a tetrameric structure in evolution. The binding process in SDH and many other tetrameric enzymes can be described by the gain in free energy which can be determined fro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
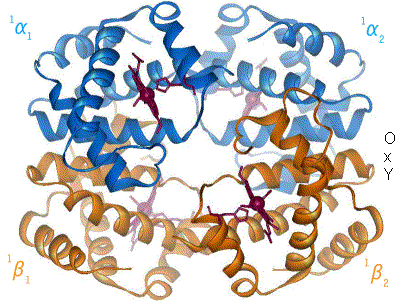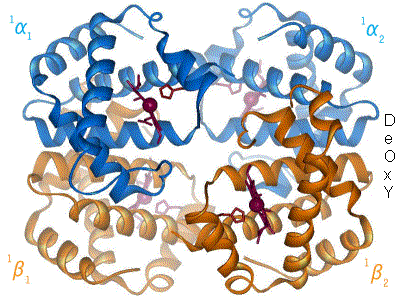
Hemoglobin
Hemoglobin (haemoglobin BrE) (from the Greek word αἷμα, ''haîma'' 'blood' + Latin ''globus'' 'ball, sphere' + ''-in'') (), abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein present in red blood cells (erythrocytes) of almost all vertebrates (the exception being the fish family Channichthyidae) as well as the tissues of some invertebrates. Hemoglobin in blood carries oxygen from the respiratory organs (''e.g.'' lungs or gills) to the rest of the body (''i.e.'' tissues). There it releases the oxygen to permit aerobic respiration to provide energy to power functions of an organism in the process called metabolism. A healthy individual human has 12to 20grams of hemoglobin in every 100mL of blood. In mammals, the chromoprotein makes up about 96% of the red blood cells' dry content (by weight), and around 35% of the total content (including water). Hemoglobin has an oxygen-binding capacity of 1.34mL O2 per gram, which increases the total blood oxygen ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tautomer
Tautomers () are structural isomers (constitutional isomers) of chemical compounds that readily interconvert. The chemical reaction interconverting the two is called tautomerization. This conversion commonly results from the relocation of a hydrogen atom within the compound. The phenomenon of tautomerization is called tautomerism, also called desmotropism. Tautomerism is for example relevant to the behavior of amino acids and nucleic acids, two of the fundamental building blocks of life. Care should be taken not to confuse tautomers with depictions of "contributing structures" in chemical resonance. Tautomers are distinct chemical species that can be distinguished by their differing atomic connectivities, molecular geometries, and physicochemical and spectroscopic properties, whereas resonance forms are merely alternative Lewis structure (valence bond theory) depictions of a single chemical species, whose true structure is best described as the "average" of the idealized, hypo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Symmetries
Symmetry (from grc, συμμετρία "agreement in dimensions, due proportion, arrangement") in everyday language refers to a sense of harmonious and beautiful proportion and balance. In mathematics, "symmetry" has a more precise definition, and is usually used to refer to an object that is invariant under some transformations; including translation, reflection, rotation or scaling. Although these two meanings of "symmetry" can sometimes be told apart, they are intricately related, and hence are discussed together in this article. Mathematical symmetry may be observed with respect to the passage of time; as a spatial relationship; through geometric transformations; through other kinds of functional transformations; and as an aspect of abstract objects, including theoretic models, language, and music. This article describes symmetry from three perspectives: in mathematics, including geometry, the most familiar type of symmetry for many people; in science and nature ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oligomeric Protein
A protein complex or multiprotein complex is a group of two or more associated polypeptide chains. Protein complexes are distinct from multienzyme complexes, in which multiple catalytic domains are found in a single polypeptide chain. Protein complexes are a form of quaternary structure. Proteins in a protein complex are linked by non-covalent protein–protein interactions. These complexes are a cornerstone of many (if not most) biological processes. The cell is seen to be composed of modular supramolecular complexes, each of which performs an independent, discrete biological function. Through proximity, the speed and selectivity of binding interactions between enzymatic complex and substrates can be vastly improved, leading to higher cellular efficiency. Many of the techniques used to enter cells and isolate proteins are inherently disruptive to such large complexes, complicating the task of determining the components of a complex. Examples of protein complexes include the p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Point Group
In geometry, a point group is a mathematical group of symmetry operations ( isometries in a Euclidean space) that have a fixed point in common. The coordinate origin of the Euclidean space is conventionally taken to be a fixed point, and every point group in dimension ''d'' is then a subgroup of the orthogonal group O(''d''). Point groups are used to describe the symmetries of geometric figures and physical objects such as molecules. Each point group can be represented as sets of orthogonal matrices ''M'' that transform point ''x'' into point ''y'' according to Each element of a point group is either a rotation (determinant of ''M'' = 1), or it is a reflection or improper rotation (determinant of ''M'' = −1). The geometric symmetries of crystals are described by space groups, which allow translations and contain point groups as subgroups. Discrete point groups in more than one dimension come in infinite families, but from the crystallographic restriction theore ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.gif)