|
Protegrin
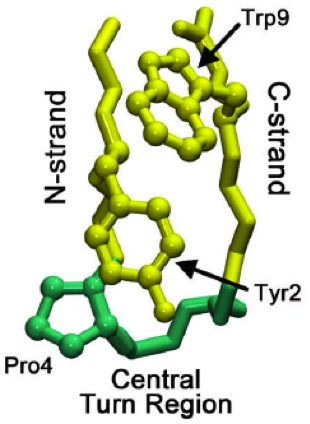
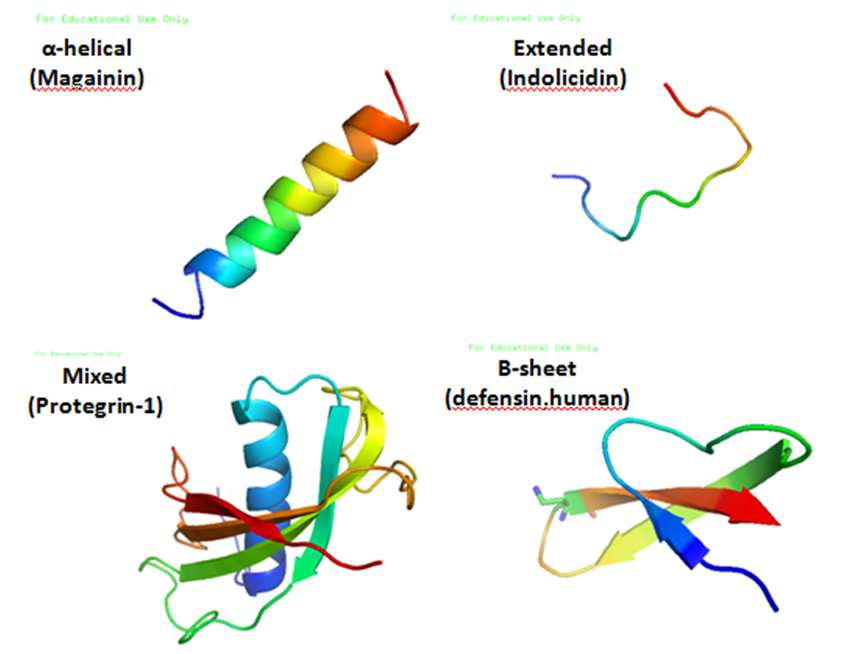
Protegrins are small peptides containing 16-18 amino acid residues. Protegrins were first discovered in porcine leukocytes and were found to have antimicrobial activity against bacteria, fungi, and some enveloped viruses. The amino acid composition of protegrins contains six positively charged arginine residues and four cysteine residues. Their secondary structure is classified as cysteine-rich β-sheet antimicrobial peptides, AMPs, that display limited sequence similarity to certain defensins and tachyplesins. In solution, the peptides fold to form an anti-parallel β-strand with the structure stabilized by two cysteine bridges formed among the four cysteine residues. Recent studies suggest that protegrins can bind to lipopolysaccharide, a property that may help them to insert into the membranes of gram-negative bacteria and permeabilize them. Structure There are five known porcine protegrins, PG-1 to PG-5. Three were identified biochemically and rest of them were deduced from D ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protegrin Structures
Protegrins are small peptides containing 16-18 amino acid residues. Protegrins were first discovered in porcine leukocytes and were found to have antimicrobial activity against bacteria, fungi, and some enveloped viruses. The amino acid composition of protegrins contains six positively charged arginine residues and four cysteine residues. Their secondary structure is classified as cysteine-rich β-sheet antimicrobial peptides, AMPs, that display limited sequence similarity to certain defensins and tachyplesins. In solution, the peptides fold to form an anti-parallel β-strand with the structure stabilized by two cysteine bridges formed among the four cysteine residues. Recent studies suggest that protegrins can bind to lipopolysaccharide, a property that may help them to insert into the membranes of gram-negative bacteria and permeabilize them. Structure There are five known porcine protegrins, PG-1 to PG-5. Three were identified biochemically and rest of them were deduced from D ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peptides
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides. A polypeptide is a longer, continuous, unbranched peptide chain. Hence, peptides fall under the broad chemical classes of biological polymers and oligomers, alongside nucleic acids, oligosaccharides, polysaccharides, and others. A polypeptide that contains more than approximately 50 amino acids is known as a protein. Proteins consist of one or more polypeptides arranged in a biologically functional way, often bound to ligands such as coenzymes and cofactors, or to another protein or other macromolecule such as DNA or RNA, or to complex macromolecular assemblies. Amino acids that have been incorporated into peptides are termed residues. A water molecule is released during formation of each amide bond.. All peptides except cyclic peptides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Defensins
Defensins are small cysteine-rich cationic proteins across cellular life, including vertebrate and invertebrate animals, plants, and fungi. They are host defense peptides, with members displaying either direct antimicrobial activity, immune signalling activities, or both. They are variously active against bacteria, fungi and many enveloped and nonenveloped viruses. They are typically 18-45 amino acids in length, with three or four highly conserved disulphide bonds. In animals, they are produced by cells of the innate immune system and epithelial cells, whereas in plants and fungi they are produced by a wide variety of tissues. An organism usually produces many different defensins, some of which are stored inside the cells (e.g. in neutrophil granulocytes to kill phagocytosed bacteria), and others are secreted into the extracellular medium. For those that directly kill microbes, their mechanism of action varies from disruption of the microbial cell membrane to metabolic disruptio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pseudomonas
''Pseudomonas'' is a genus of Gram-negative, Gammaproteobacteria, belonging to the family Pseudomonadaceae and containing 191 described species. The members of the genus demonstrate a great deal of metabolic diversity and consequently are able to colonize a wide range of niches. Their ease of culture ''in vitro'' and availability of an increasing number of ''Pseudomonas'' strain genome sequences has made the genus an excellent focus for scientific research; the best studied species include ''P. aeruginosa'' in its role as an opportunistic human pathogen, the plant pathogen '' P. syringae'', the soil bacterium '' P. putida'', and the plant growth-promoting ''P. fluorescens, P. lini, P. migulae'', and ''P. graminis''. Because of their widespread occurrence in water and plant seeds such as dicots, the pseudomonads were observed early in the history of microbiology. The generic name ''Pseudomonas'' created for these organisms was defined in rather vague terms by Walter Migula i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta Hairpin
The beta hairpin (sometimes also called beta-ribbon or beta-beta unit) is a simple protein structural motif involving two beta strands that look like a hairpin. The motif consists of two strands that are adjacent in primary structure, oriented in an antiparallel direction (the N-terminus of one sheet is adjacent to the C-terminus of the next), and linked by a short loop of two to five amino acids. Beta hairpins can occur in isolation or as part of a series of hydrogen bonded strands that collectively comprise a beta sheet. Researchers such as Francisco Blanco ''et al.'' have used protein NMR to show that beta-hairpins can be formed from isolated short peptides in aqueous solution, suggesting that hairpins could form nucleation sites for protein folding. Classification Beta hairpins were originally categorized solely by the number of amino acid residues in their loop sequences, such that they were named one-residue, two-residue, etc. This system, however, is somewhat ambiguous ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
University Of Zurich
The University of Zürich (UZH, german: Universität Zürich) is a public research university located in the city of Zürich, Switzerland. It is the largest university in Switzerland, with its 28,000 enrolled students. It was founded in 1833 from the existing colleges of theology, law, medicine which go back to 1525, and a new faculty of philosophy. Currently, the university has seven faculties: Philosophy, Human Medicine, Economic Sciences, Law, Mathematics and Natural Sciences, Theology and Veterinary Medicine. The university offers the widest range of subjects and courses of any Swiss higher education institution. History The University of Zurich was founded on April 29, 1833, when the existing colleges of theology, the ''Carolinum'' founded by Huldrych Zwingli in 1525, law and medicine were merged with a new faculty of Philosophy. It was the first university in Europe to be founded by the state rather than a monarch or church. In the university's early years, the 183 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peptidomimetic
A peptidomimetic is a small protein-like chain designed to mimic a peptide. They typically arise either from modification of an existing peptide, or by designing similar systems that mimic peptides, such as peptoids and Beta-peptide, β-peptides. Irrespective of the approach, the altered chemical structure is designed to advantageously adjust the molecular properties such as stability or biological activity. This can have a role in the development of drug-like compounds from existing peptides. Peptidomimetics can be prepared by cyclization of linear peptides or coupling of stable unnatural amino acids. These modifications involve changes to the peptide that will not occur naturally (such as altered backbones and the incorporation of nonnatural amino acids). Unnatural amino acids can be generated from their native analogs via modifications such as amine alkylation, side chain substitution, structural bond extension cyclization, and isosteric replacements within the amino acid backb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gram-negative Bacteria
Gram-negative bacteria are bacteria that do not retain the crystal violet stain used in the Gram staining method of bacterial differentiation. They are characterized by their cell envelopes, which are composed of a thin peptidoglycan cell wall sandwiched between an inner cytoplasmic cell membrane and a bacterial outer membrane. Gram-negative bacteria are found in virtually all environments on Earth that support life. The gram-negative bacteria include the model organism ''Escherichia coli'', as well as many pathogenic bacteria, such as ''Pseudomonas aeruginosa'', '' Chlamydia trachomatis'', and ''Yersinia pestis''. They are a significant medical challenge as their outer membrane protects them from many antibiotics (including penicillin), detergents that would normally damage the inner cell membrane, and lysozyme, an antimicrobial enzyme produced by animals that forms part of the innate immune system. Additionally, the outer leaflet of this membrane comprises a complex lipopol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lipopolysaccharide
Lipopolysaccharides (LPS) are large molecules consisting of a lipid and a polysaccharide that are bacterial toxins. They are composed of an O-antigen, an outer core, and an inner core all joined by a covalent bond, and are found in the outer membrane of Gram-negative bacteria. Today, the term ''endotoxin'' is often used synonymously with LPS, although there are a few endotoxins (in the original sense of toxins that are inside the bacterial cell that are released when the cell disintegrates) that are not related to LPS, such as the so-called delta endotoxin proteins produced by '' Bacillus thuringiensis''. Lipopolysaccharides can have substantial impacts on human health, primarily through interactions with the immune system. LPS is a potent activator of the immune system and pyrogen (agent that causes fever). In severe cases, LPS can play a role in causing septic shock. In lower levels and over a longer time period, there is evidence LPS may play an important and harmful role ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antimicrobial Peptides
Antimicrobial peptides (AMPs), also called host defence peptides (HDPs) are part of the innate immune response found among all classes of life. Fundamental differences exist between prokaryotic and eukaryotic cells that may represent targets for antimicrobial peptides. These peptides are potent, broad spectrum antibiotics which demonstrate potential as novel therapeutic agents. Antimicrobial peptides have been demonstrated to kill Gram negative and Gram positive bacteria, enveloped viruses, fungi and even transformed or cancerous cells. Unlike the majority of conventional antibiotics it appears that antimicrobial peptides frequently destabilize biological membranes, can form transmembrane channels, and may also have the ability to enhance immunity by functioning as immunomodulators. Structure Antimicrobial peptides are a unique and diverse group of molecules, which are divided into subgroups on the basis of their amino acid composition and structure. Antimicrobial peptides are g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha amino acids appear in the genetic code. Amino acids can be classified according to the locations of the core structural functional groups, as Alpha and beta carbon, alpha- , beta- , gamma- or delta- amino acids; other categories relate to Chemical polarity, polarity, ionization, and side chain group type (aliphatic, Open-chain compound, acyclic, aromatic, containing hydroxyl or sulfur, etc.). In the form of proteins, amino acid '' residues'' form the second-largest component (water being the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling life ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |