|
Propyl Alcohol
There are two isomers of propanol. * 1-Propanol, ''n''-propanol, or propan-1-ol : CH3CH2CH2OH, the most common meaning *2-Propanol, Isopropyl alcohol, isopropanol, or propan-2-ol : (CH3)2CHOH See also * Propanal (propionaldehyde) differs in spelling from propanol by a single letter and is a different compound. * Propranolol Propranolol, sold under the brand name Inderal among others, is a medication of the beta blocker class. It is used to treat high blood pressure, a number of types of irregular heart rate, thyrotoxicosis, capillary hemangiomas, performance an ... is a drug used for reducing blood pressure and hand tremors. {{Authority control Alkanols ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers. Isomers do not necessarily share similar chemical or physical properties. Two main forms of isomerism are structural or constitutional isomerism, in which ''bonds'' between the atoms differ; and stereoisomerism or spatial isomerism, in which the bonds are the same but the ''relative positions'' of the atoms differ. Isomeric relationships form a hierarchy. Two chemicals might be the same constitutional isomer, but upon deeper analysis be stereoisomers of each other. Two molecules that are the same stereoisomer as each other might be in different conformational forms or be different isotopologues. The depth of analysis depends on the field of study or the chemical and physical properties of interest. The English word "isomer" () is a back-for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1-Propanol
Propan-1-ol (also propanol, n-propyl alcohol) is a primary alcohol with the formula and sometimes represented as PrOH or ''n''-PrOH. It is a colorless liquid and an isomer of 2-propanol. It is formed naturally in small amounts during many fermentation processes and used as a solvent in the pharmaceutical industry, mainly for resins and cellulose esters, and, sometimes, as a disinfecting agent. Chemical properties Propan-1-ol shows the normal reactions of a primary alcohol. Thus it can be converted to alkyl halides; for example red phosphorus and iodine produce n-propyl iodide in 80% yield, while with catalytic gives n-propyl chloride. Reaction with acetic acid in the presence of an catalyst under Fischer esterification conditions gives propyl acetate, while refluxing propanol overnight with formic acid alone can produce propyl formate in 65% yield. Oxidation of propan-1-ol with and gives a 36% yield of propionaldehyde, and therefore for this type of reaction higher yi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isopropyl Alcohol
Isopropyl alcohol (IUPAC name propan-2-ol and also called isopropanol or 2-propanol) is a colorless, flammable organic compound with a pungent alcoholic odor. As an isopropyl group linked to a hydroxyl group (chemical formula ) it is the simplest example of a secondary alcohol, where the alcohol carbon atom is attached to two other carbon atoms. It is a structural isomer of propan-1-ol and ethyl methyl ether. It is used in the manufacture of a wide variety of industrial and household chemicals and is a common ingredient in products such as antiseptics, disinfectants, hand sanitizer and detergents. Well over one million tonnes is produced worldwide annually. Properties Isopropyl alcohol is miscible in water, ethanol, and chloroform as, like these compounds, isopropyl is a polar molecule. It dissolves ethyl cellulose, polyvinyl butyral, many oils, alkaloids, and natural resins. Unlike ethanol or methanol, isopropyl alcohol is not miscible with salt solutions and can be separ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propionaldehyde
Propionaldehyde or propanal is the organic compound with the formula CH3CH2CHO. It is the 3-carbon aldehyde. It is a colourless, flammable liquid with a slightly fruity odour. It is produced on a large scale industrially. Production Propionaldehyde is mainly produced industrially by hydroformylation of ethylene: :CO + H2 + C2H4 → CH3CH2CHO In this way, several hundred thousand tons are produced annually. Laboratory preparation Propionaldehyde may also be prepared by oxidizing 1-propanol with a mixture of sulfuric acid and potassium dichromate. The reflux condenser contains water heated at 60 °C, which condenses unreacted propanol, but allows propionaldehyde to pass. The propionaldehyde vapor is immediately condensed into a suitable receiver. In this arrangement, any propionaldehyde formed is immediately removed from the reactor, thus it does not get over-oxidized to propionic acid. Reactions Propionaldehyde exhibits the reactions characteristic of alkyl aldehydes, e.g. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
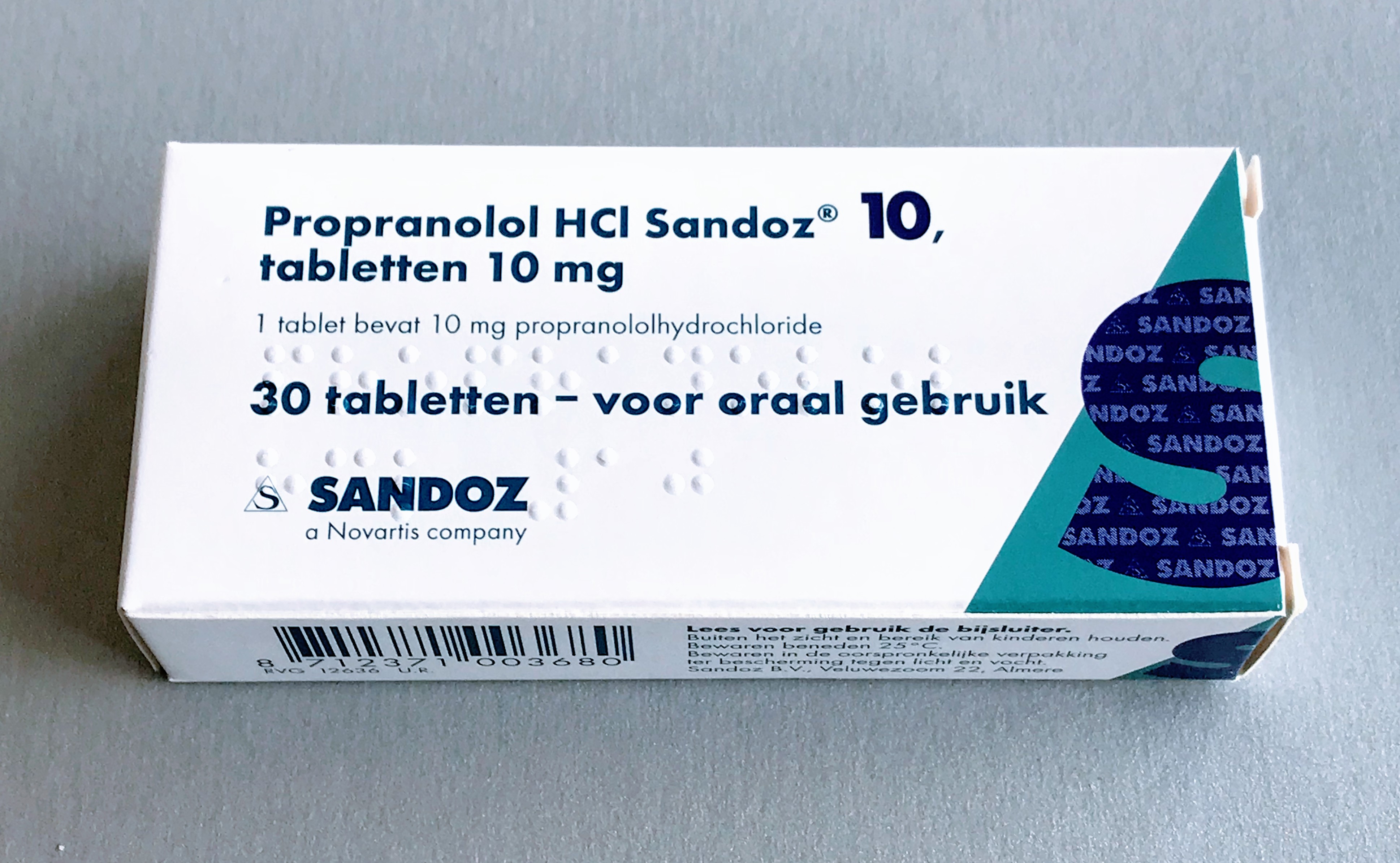
Propranolol
Propranolol, sold under the brand name Inderal among others, is a medication of the beta blocker class. It is used to treat high blood pressure, a number of types of irregular heart rate, thyrotoxicosis, capillary hemangiomas, performance anxiety, and essential tremors, as well to prevent migraine headaches, and to prevent further heart problems in those with angina or previous heart attacks. It can be taken by mouth or by injection into a vein. The formulation that is taken by mouth comes in short-acting and long-acting versions. Propranolol appears in the blood after 30 minutes and has a maximum effect between 60 and 90 minutes when taken by mouth. Common side effects include nausea, abdominal pain, and constipation. It should not be used in those with an already slow heart rate and most of those with heart failure. Quickly stopping the medication in those with coronary artery disease may worsen symptoms. It may worsen the symptoms of asthma. Caution is recommended in those ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |