|
Preclinical
In drug development, preclinical development, also termed preclinical studies or nonclinical studies, is a stage of research that begins before clinical trials (testing in humans) and during which important feasibility, iterative testing and drug safety data are collected, typically in laboratory animals. The main goals of preclinical studies are to determine a starting, safe dose for first-in-human study and assess potential toxicity of the product, which typically include new medical devices, prescription drugs, and diagnostics. Companies use stylized statistics to illustrate the risks in preclinical research, such as that on average, only one in every 5,000 compounds that enters drug discovery to the stage of preclinical development becomes an approved drug. Types of preclinical research Each class of product may undergo different types of preclinical research. For instance, drugs may undergo pharmacodynamics (what the drug does to the body) (PD), pharmacokinetics (wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drug Development
Drug development is the process of bringing a new pharmaceutical drug to the market once a lead compound has been identified through the process of drug discovery. It includes preclinical research on microorganisms and animals, filing for regulatory status, such as via the United States Food and Drug Administration for an investigational new drug to initiate clinical trials on humans, and may include the step of obtaining regulatory approval with a new drug application to market the drug. The entire process – from concept through preclinical testing in the laboratory to clinical trial development, including Phase I–III trials – to approved vaccine or drug typically takes more than a decade. New chemical entity development Broadly, the process of drug development can be divided into preclinical and clinical work. Pre-clinical New chemical entities (NCEs, also known as new molecular entities or NMEs) are compounds that emerge from the process of drug discovery. T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
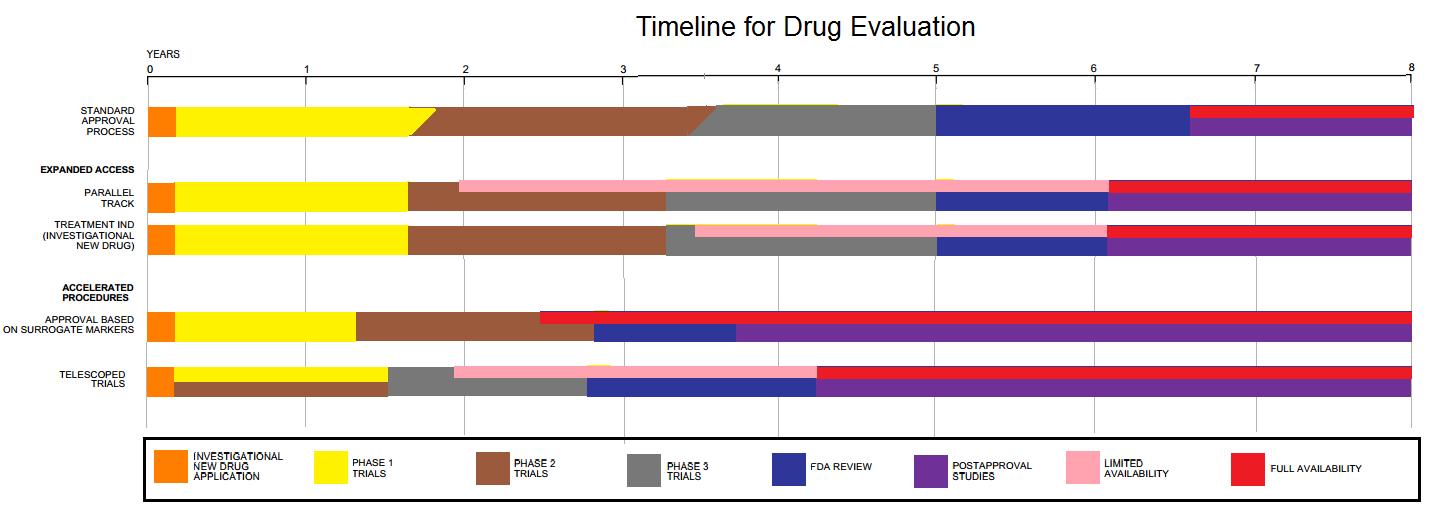
Phases Of Clinical Research
The phases of clinical research are the stages in which scientists conduct experiments with a health intervention to obtain sufficient evidence for a process considered effective as a medical treatment. For drug development, the clinical phases start with testing for safety in a few human subjects, then expand to many study participants (potentially tens of thousands) to determine if the treatment is effective. Clinical research is conducted on drug candidates, vaccine candidates, new medical devices, and new diagnostic assays. Summary Clinical trials testing potential medical products are commonly classified into four phases. The drug development process will normally proceed through all four phases over many years. If the drug successfully passes through Phases I, II, and III, it will usually be approved by the national regulatory authority for use in the general population. Phase IV trials are 'post-marketing' or 'surveillance' studies conducted to monitor safety over sever ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Toxicology Testing
Toxicology testing, also known as safety assessment, or toxicity testing, is the process of determining the degree to which a substance of interest negatively impacts the normal biological functions of an organism, given a certain exposure duration, route of exposure, and substance concentration. Toxicology testing is often conducted by researchers who follow established toxicology test protocol for a certain substance, mode of exposure, exposure environment, duration of exposure, or for a particular organism of interest, or for a particular developmental stage of interest. Toxicology testing is commonly conducted during preclinical development for a substance intended for human exposure. Stages of ''in silico'', ''in vitro'' and ''in vivo'' research are conducted to determine safe exposure doses in model organisms. If necessary, the next phase of research involves human toxicology testing during a first-in-man study. Toxicology testing may be conducted by the pharmaceutical indu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Approved Drug
An approved drug is a Medicine, medicinal preparation that has been validated for a therapeutic use by a Regulation of therapeutic goods, ruling authority of a government. This process is usually specific by country, unless specified otherwise. Process by country United States In the United States, the Food and Drug Administration, FDA approves medication, drugs. Before a drug can be prescribed, it must undergo the FDA's approval process. While a drug can feasibly be used off-label (for non-approved indications), it still is required to be approved for a specific disease or medical condition. Drug companies seeking to sell a drug in the United States must first test it. The company then sends the Food and Drug Administration's Center for Drug Evaluation and Research (CDER) evidence from these tests to prove the drug is safe and effective for its intended use. A fee is required to make such FDA submission. For financial year 2020, this fee was: for an application requiring clinic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Toxicity
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a substructure of the organism, such as a cell ( cytotoxicity) or an organ such as the liver (hepatotoxicity). By extension, the word may be metaphorically used to describe toxic effects on larger and more complex groups, such as the family unit or society at large. Sometimes the word is more or less synonymous with poisoning in everyday usage. A central concept of toxicology is that the effects of a toxicant are dose-dependent; even water can lead to water intoxication when taken in too high a dose, whereas for even a very toxic substance such as snake venom there is a dose below which there is no detectable toxic effect. Toxicity is species-specific, making cross-species analysis problematic. Newer paradigms and metrics are evolving to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cancer
Cancer is a group of diseases involving abnormal cell growth with the potential to invade or spread to other parts of the body. These contrast with benign tumors, which do not spread. Possible signs and symptoms include a lump, abnormal bleeding, prolonged cough, unexplained weight loss, and a change in bowel movements. While these symptoms may indicate cancer, they can also have other causes. Over 100 types of cancers affect humans. Tobacco use is the cause of about 22% of cancer deaths. Another 10% are due to obesity, poor diet, lack of physical activity or excessive drinking of alcohol. Other factors include certain infections, exposure to ionizing radiation, and environmental pollutants. In the developing world, 15% of cancers are due to infections such as '' Helicobacter pylori'', hepatitis B, hepatitis C, human papillomavirus infection, Epstein–Barr virus and human immunodeficiency virus (HIV). These factors act, at least partly, by changing the genes o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
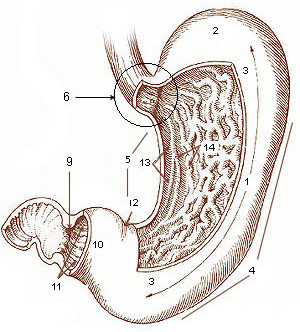
Gastric
The stomach is a muscular, hollow organ in the gastrointestinal tract of humans and many other animals, including several invertebrates. The stomach has a dilated structure and functions as a vital organ in the digestive system. The stomach is involved in the gastric phase of digestion, following chewing. It performs a chemical breakdown by means of enzymes and hydrochloric acid. In humans and many other animals, the stomach is located between the oesophagus and the small intestine. The stomach secretes digestive enzymes and gastric acid to aid in food digestion. The pyloric sphincter controls the passage of partially digested food ( chyme) from the stomach into the duodenum, where peristalsis takes over to move this through the rest of intestines. Structure In the human digestive system, the stomach lies between the oesophagus and the duodenum (the first part of the small intestine). It is in the left upper quadrant of the abdominal cavity. The top of the stomach lies ag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adverse Effect (medicine)
An adverse effect is an undesired harmful effect resulting from a medication or other intervention, such as surgery. An adverse effect may be termed a " side effect", when judged to be secondary to a main or therapeutic effect. The term complication is similar to adverse effect, but the latter is typically used in pharmacological contexts, or when the negative effect is expected or common. If the negative effect results from an unsuitable or incorrect dosage or procedure, this is called a medical error and not an adverse effect. Adverse effects are sometimes referred to as " iatrogenic" because they are generated by a physician/treatment. Some adverse effects occur only when starting, increasing or discontinuing a treatment. Adverse effects can also be caused by placebo treatments (in which case the adverse effects are referred to as nocebo effects). Using a drug or other medical intervention which is contraindicated may increase the risk of adverse effects. Adverse effe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metabolites
In biochemistry, a metabolite is an intermediate or end product of metabolism. The term is usually used for small molecules. Metabolites have various functions, including fuel, structure, signaling, stimulatory and inhibitory effects on enzymes, catalytic activity of their own (usually as a cofactor to an enzyme), defense, and interactions with other organisms (e.g. pigments, odorants, and pheromones). A primary metabolite is directly involved in normal "growth", development, and reproduction. Ethylene exemplifies a primary metabolite produced large-scale by industrial microbiology. A secondary metabolite is not directly involved in those processes, but usually has an important ecological function. Examples include antibiotics and pigments such as resins and terpenes etc. Some antibiotics use primary metabolites as precursors, such as actinomycin, which is created from the primary metabolite tryptophan. Some sugars are metabolites, such as fructose or glucose, which are bo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
In Vivo
Studies that are ''in vivo'' (Latin for "within the living"; often not italicized in English) are those in which the effects of various biological entities are tested on whole, living organisms or cells, usually animals, including humans, and plants, as opposed to a tissue extract or dead organism. This is not to be confused with experiments done ''in vitro'' ("within the glass"), i.e., in a laboratory environment using test tubes, Petri dishes, etc. Examples of investigations ''in vivo'' include: the pathogenesis of disease by comparing the effects of bacterial infection with the effects of purified bacterial toxins; the development of non-antibiotics, antiviral drugs, and new drugs generally; and new surgical procedures. Consequently, animal testing and clinical trials are major elements of ''in vivo'' research. ''In vivo'' testing is often employed over ''in vitro'' because it is better suited for observing the overall effects of an experiment on a living subject. In dr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dosage Form
Dosage forms (also called unit doses) are pharmaceutical drug products in the form in which they are marketed for use, with a specific mixture of active ingredients and inactive components ( excipients), in a particular configuration (such as a capsule shell, for example), and apportioned into a particular dose. For example, two products may both be amoxicillin, but one is in 500 mg capsules and another is in 250 mg chewable tablets. The term unit dose can also sometimes encompass non-reusable ''packaging'' as well (especially when each drug product is individually packaged), although the FDA distinguishes that by ''unit-dose "packaging" or "dispensing"''. Depending on the context, ''multi(ple) unit dose'' can refer to distinct drug products ''packaged'' together, or to a ''single'' drug product containing multiple drugs and/or doses. The term dosage form can also sometimes refer ''only'' to the pharmaceutical formulation of a drug product's constituent drug substance(s) and any ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Circulatory System
The blood circulatory system is a system of organs that includes the heart, blood vessels, and blood which is circulated throughout the entire body of a human or other vertebrate. It includes the cardiovascular system, or vascular system, that consists of the heart and blood vessels (from Greek ''kardia'' meaning ''heart'', and from Latin ''vascula'' meaning ''vessels''). The circulatory system has two divisions, a systemic circulation or circuit, and a pulmonary circulation or circuit. Some sources use the terms ''cardiovascular system'' and ''vascular system'' interchangeably with the ''circulatory system''. The network of blood vessels are the great vessels of the heart including large elastic arteries, and large veins; other arteries, smaller arterioles, capillaries that join with venules (small veins), and other veins. The circulatory system is closed in vertebrates, which means that the blood never leaves the network of blood vessels. Some invertebrates such as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.jpg)