|
Potash Feldspar
Orthoclase, or orthoclase feldspar (endmember formula K Al Si3 O8), is an important tectosilicate mineral which forms igneous rock. The name is from the Ancient Greek for "straight fracture," because its two cleavage planes are at right angles to each other. It is a type of potassium feldspar, also known as K-feldspar. The gem known as moonstone (see below) is largely composed of orthoclase. Formation and subtypes Orthoclase is a common constituent of most granites and other felsic igneous rocks and often forms huge crystals and masses in pegmatite. Typically, the pure potassium endmember of orthoclase forms a solid solution with albite, the sodium endmember (NaAlSi3O8), of plagioclase. While slowly cooling within the earth, sodium-rich albite lamellae form by exsolution, enriching the remaining orthoclase with potassium. The resulting intergrowth of the two feldspars is called perthite. The higher-temperature polymorph of KAlSi3O8 is sanidine. Sanidine is common in rapidly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicate Mineral
Silicate minerals are rock-forming minerals made up of silicate groups. They are the largest and most important class of minerals and make up approximately 90 percent of Earth's crust. In mineralogy, silica (silicon dioxide, ) is usually considered a silicate mineral. Silica is found in nature as the mineral quartz, and its polymorphism (materials science), polymorphs. On Earth, a wide variety of silicate minerals occur in an even wider range of combinations as a result of the processes that have been forming and re-working the crust for billions of years. These processes include partial melting, crystallization, fractionation, metamorphism, weathering, and diagenesis. Living organisms also contribute to this carbonate–silicate cycle, geologic cycle. For example, a type of plankton known as diatoms construct their exoskeletons ("frustules") from silica extracted from seawater. The frustules of dead diatoms are a major constituent of deep ocean sediment, and of diatomaceous e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Moonstone (gemstone)
Moonstone is a sodium potassium aluminium silicate ((Na,K)AlSi3O8) of the feldspar group that displays a pearly and opalescent schiller. An alternative name is hecatolite. Etymology File:Pierrelune.jpg, Moonstone polished ''en cabochon'' The name ''moonstone'' derives from the stone's characteristic visual effect, called adularescence (or schiller), which produces a milky, bluish interior light. This effect is caused by light diffraction through alternating layers of orthoclast and albite within the stone. The diffracted light varies from white to blue, depending on the thinness of the albite layers. More technically, this micro-structure consists of regular exsolution layers (lamellae) of different alkali feldspars (orthoclase and sodium-rich plagioclase). Polished moonstones often display chatoyancy ("cat's eye" effect), where a luminous streak appears through the stone. Asterism is rare and produces four-legged stars. Geology The most common moonstone is of the orthocl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Obsidian
Obsidian () is a naturally occurring volcanic glass formed when lava extrusive rock, extruded from a volcano cools rapidly with minimal crystal growth. It is an igneous rock. Obsidian is produced from felsic lava, rich in the lighter elements such as silicon, oxygen, aluminium, sodium, and potassium. It is commonly found within the margins of rhyolite, rhyolitic lava flows known as obsidian flows. These flows have a high content of silicon dioxide, silica, granting them a high viscosity. The high viscosity inhibits atomic diffusion, diffusion of atoms through the lava, which inhibits the first step (nucleation) in the formation of mineral crystals. Together with rapid cooling, this results in a natural glass forming from the lava. Obsidian is hard, Brittleness, brittle, and amorphous; it therefore Fracture (mineralogy)#Conchoidal fracture, fractures with sharp edges. In the past, it was used to manufacture cutting and piercing tools, and it has been used experimentally as surgic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sanidine
Sanidine is the high temperature form of potassium feldspar with a general formula K(AlSi3O8). Sanidine is found most typically in felsic volcanic rocks such as obsidian, rhyolite and trachyte. Sanidine crystallizes in the monoclinic crystal system. Orthoclase is a monoclinic polymorph stable at lower temperatures. At yet lower temperatures, microcline, a triclinic polymorph of potassium feldspar, is stable. Due to the high temperature and rapid quenching, sanidine can contain more sodium in its structure than the two polymorphs that equilibrated at lower temperatures. Sanidine and high albite constitute a solid solution series with intermediate compositions termed anorthoclase. Exsolution of an albite phase does occur; resulting cryptoperthite can best be observed in electron microprobe images. Occurrence In addition to its presence in the groundmass of felsic rocks, sanidine is a common phenocryst in rhyolites and, to a lesser extent, rhyodacites. Trachyte consists largely of fi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymorphism (materials Science)
In materials science, polymorphism describes the existence of a solid material in more than one form or crystal structure. Polymorphism is a form of isomerism. Any crystalline material can exhibit the phenomenon. Allotropy refers to polymorphism for chemical elements. Polymorphism is of practical relevance to pharmaceuticals, agrochemicals, pigments, dyestuffs, foods, and explosives. According to IUPAC, a polymorphic transition is "A reversible transition of a solid crystalline phase at a certain temperature and pressure (the inversion point) to another phase of the same chemical composition with a different crystal structure." According to McCrone, polymorphs are "different in crystal structure but identical in the liquid or vapor states." Materials with two polymorphs are called dimorphic, with three polymorphs, trimorphic, etc. Examples Many compounds exhibit polymorphism. It has been claimed that "every compound has different polymorphic forms, and that, in general, the n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adularia With Pyrite Mg 7940
Orthoclase, or orthoclase feldspar (endmember formula K Al Si3 O8), is an important tectosilicate mineral which forms igneous rock. The name is from the Ancient Greek for "straight fracture," because its two cleavage planes are at right angles to each other. It is a type of potassium feldspar, also known as K-feldspar. The gem known as moonstone (see below) is largely composed of orthoclase. Formation and subtypes Orthoclase is a common constituent of most granites and other felsic igneous rocks and often forms huge crystals and masses in pegmatite. Typically, the pure potassium endmember of orthoclase forms a solid solution with albite, the sodium endmember (NaAlSi3O8), of plagioclase. While slowly cooling within the earth, sodium-rich albite lamellae form by exsolution, enriching the remaining orthoclase with potassium. The resulting intergrowth of the two feldspars is called perthite. The higher-temperature polymorph of KAlSi3O8 is sanidine. Sanidine is common in rapidly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
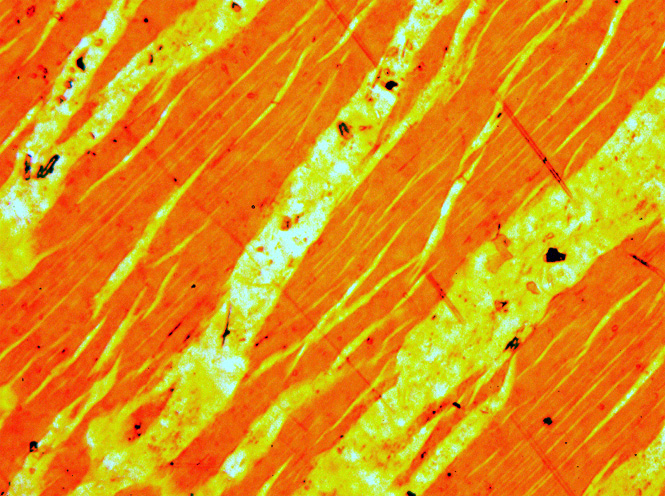
Perthite
Perthite is used to describe an intergrowth of two feldspars: a host grain of potassium-rich alkali feldspar (near K-feldspar, KAlSi3O8, in composition) includes exsolved lamellae or irregular intergrowths of sodic alkali feldspar (near albite, NaAlSi3O8, in composition). Typically, the host grain is orthoclase or microcline, and the lamellae are albite. If sodic feldspar is the dominant phase, the result is an antiperthite and where the feldspars are in roughly equal proportions the result is a mesoperthite. The intergrowth forms by exsolution due to cooling of a grain of alkali feldspar with a composition intermediate between K-feldspar and albite. There is complete solid solution between albite and K-feldspar at temperatures near 700 °C and pressures like those within the crust of the Earth, but a miscibility gap is present at lower temperatures. If an alkali feldspar grain with an intermediate composition cools slowly enough, K-rich and more Na-rich feldspar domains separ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Exsolution
A solid solution, a term popularly used for metals, is a homogenous mixture of two different kinds of atoms in solid state and have a single crystal structure. Many examples can be found in metallurgy, geology, and solid-state chemistry. The word "solution" is used to describe the intimate mixing of components at the atomic level and distinguishes these homogeneous materials from physical mixtures of components. Two terms are mainly associated with solid solutions - ''solvents'' and ''solutes,'' depending on the relative abundance of the atomic species. In general if two compounds are isostructural then a solid solution will exist between the end members (also known as parents). For example sodium chloride and potassium chloride have the same cubic crystal structure so it is possible to make a pure compound with any ratio of sodium to potassium (Na1-xKx)Cl by dissolving that ratio of NaCl and KCl in water and then evaporating the solution. A member of this family is sold under t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lamella (materials)
A ''lamella'' (plural ''lamellae'') is a small plate or flake, from the Latin, and may also be used to refer to collections of fine sheets of material held adjacent to one another, in a gill-shaped structure, often with fluid in between though sometimes simply a set of 'welded' plates. The term is used in biological contexts to describe thin membranes of plates of tissue. In context of materials science, the microscopic structures in bone and nacre are called lamellae. Moreover, the term lamella is often used as a way to describe crystal structure of some materials. Uses of the term In surface chemistry (especially mineralogy and materials science), lamellar structures are fine layers, alternating between different materials. They can be produced by chemical effects (as in eutectic solidification), biological means, or a deliberate process of lamination, such as pattern welding. Lamellae can also describe the layers of atoms in the crystal lattices of materials such as me ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plagioclase
Plagioclase is a series of tectosilicate (framework silicate) minerals within the feldspar group. Rather than referring to a particular mineral with a specific chemical composition, plagioclase is a continuous solid solution series, more properly known as the plagioclase feldspar series. This was first shown by the German mineralogist Johann Friedrich Christian Hessel (1796–1872) in 1826. The series ranges from albite to anorthite endmembers (with respective compositions NaAlSi3O8 to CaAl2Si2O8), where sodium and calcium atoms can substitute for each other in the mineral's crystal lattice structure. Plagioclase in hand samples is often identified by its polysynthetic crystal twinning or 'record-groove' effect. Plagioclase is a major constituent mineral in the Earth's crust, and is consequently an important diagnostic tool in petrology for identifying the composition, origin and evolution of igneous rocks. Plagioclase is also a major constituent of rock in the highlan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Albite
Albite is a plagioclase feldspar mineral. It is the sodium endmember of the plagioclase solid solution series. It represents a plagioclase with less than 10% anorthite content. The pure albite endmember has the formula . It is a tectosilicate. Its color is usually pure white, hence its name from Latin, . It is a common constituent in felsic rocks. Properties Albite crystallizes with triclinic pinacoidal forms. Its specific gravity is about 2.62 and it has a Mohs hardness of 6–6.5. Albite almost always exhibits crystal twinning often as minute parallel striations on the crystal face. Albite often occurs as fine parallel segregations alternating with pink microcline in perthite as a result of exolution on cooling. There are two variants of albite, which are referred to as 'low albite' and 'high albite'; the latter is also known as 'analbite'. Although both variants are triclinic, they differ in the volume of their unit cell, which is slightly larger for the 'high' form. The ' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solid Solution
A solid solution, a term popularly used for metals, is a homogenous mixture of two different kinds of atoms in solid state and have a single crystal structure. Many examples can be found in metallurgy, geology, and solid-state chemistry. The word "solution" is used to describe the intimate mixing of components at the atomic level and distinguishes these homogeneous materials from physical mixtures of components. Two terms are mainly associated with solid solutions - ''solvents'' and ''solutes,'' depending on the relative abundance of the atomic species. In general if two compounds are isostructural then a solid solution will exist between the end members (also known as parents). For example sodium chloride and potassium chloride have the same cubic crystal structure so it is possible to make a pure compound with any ratio of sodium to potassium (Na1-xKx)Cl by dissolving that ratio of NaCl and KCl in water and then evaporating the solution. A member of this family is sold under t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |