|
Phytoestrogens
A phytoestrogen is a plant-derived xenoestrogen (see estrogen) not generated within the endocrine system, but consumed by eating plants or manufactured foods. Also called a "dietary estrogen", it is a diverse group of naturally occurring nonsteroidal plant compounds that, because of its structural similarity with estradiol (17-β-estradiol), have the ability to cause estrogenic or antiestrogenic effects. Phytoestrogens are not essential nutrients because their absence from the diet does not cause a disease, nor are they known to participate in any normal biological function. Common foods containing phytoestrogens are soy protein, beans, oats, barley, rice, coffee, apples, carrots (see Food Sources section below for bigger list). Its name comes from the Greek ''phyto'' ("plant") and ''estrogen'', the hormone which gives fertility to female mammals. The word "estrus" - Greek οίστρος - means "sexual desire", and "gene" - Greek γόνο - is "to generate". It has been hypoth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phytoestrogens2
A phytoestrogen is a plant-derived xenoestrogen (see estrogen) not generated within the endocrine system, but consumed by eating plants or manufactured foods. Also called a "dietary estrogen", it is a diverse group of naturally occurring nonsteroidal plant compounds that, because of its structural similarity with estradiol (17-β-estradiol), have the ability to cause estrogenic or antiestrogenic effects. Phytoestrogens are not essential nutrients because their absence from the diet does not cause a disease, nor are they known to participate in any normal biological function. Common foods containing phytoestrogens are soy protein, beans, oats, barley, rice, coffee, apples, carrots (see Food Sources section below for bigger list). Its name comes from the Greek ''phyto'' ("plant") and ''estrogen'', the hormone which gives fertility to female mammals. The word "estrus" - Greek οίστρος - means "sexual desire", and "gene" - Greek γόνο - is "to generate". It has been hypoth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
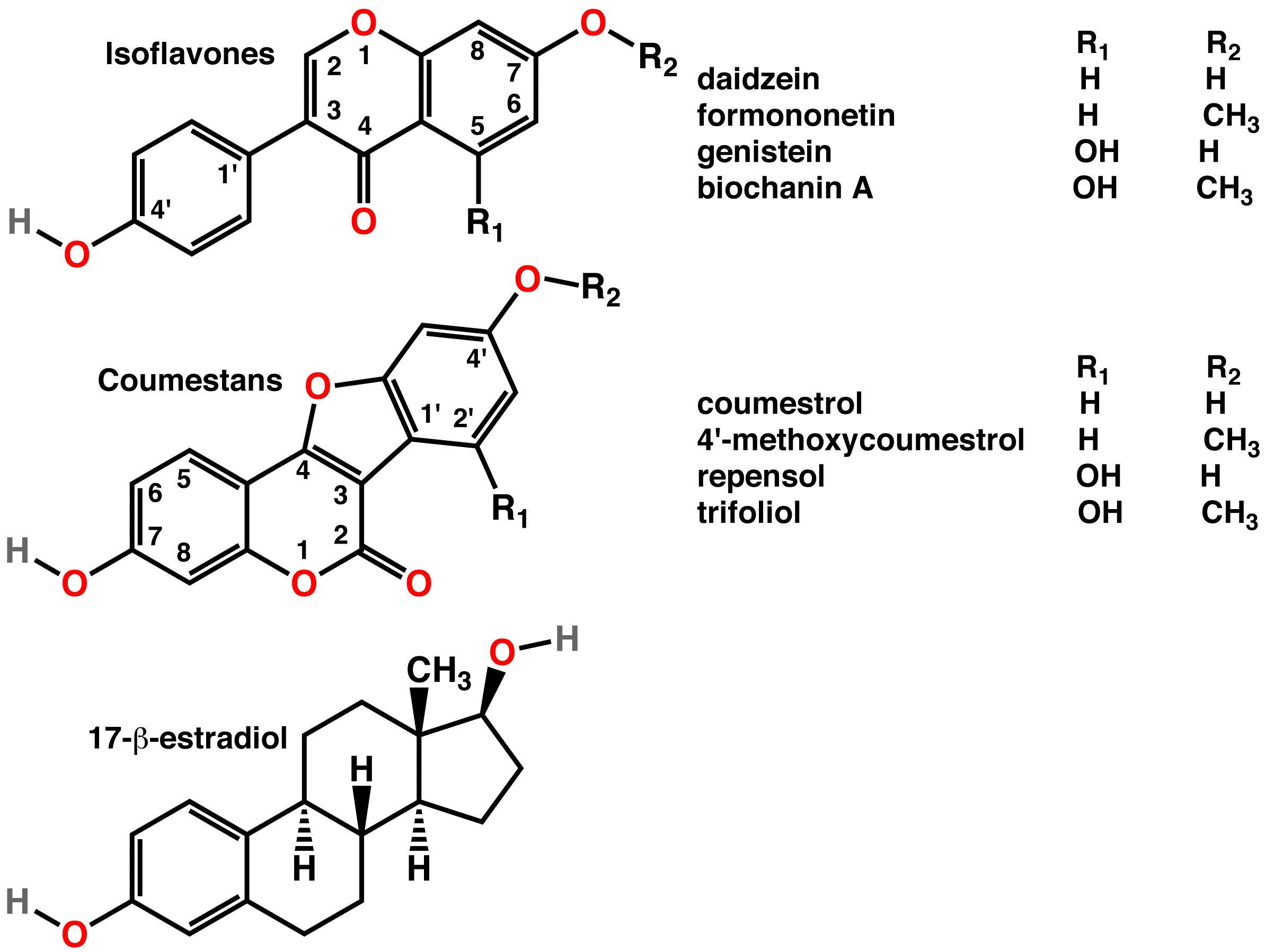
Isoflavone
Isoflavones are substituted derivatives of isoflavone, a type of naturally occurring isoflavonoids, many of which act as phytoestrogens in mammals. Isoflavones are produced almost exclusively by the members of the bean family, Fabaceae (Leguminosae). Although isoflavones and closely related phytoestrogens are sold as dietary supplements, there is little scientific evidence for either the safety of long-term supplementation or of health benefits from these compounds. Some studies have identified potential risks from high intake of isoflavones, such as in women with a history of breast cancer, but this concern has not been substantiated with high-quality clinical research. Organic chemistry and biosynthesis Isoflavone is an isomer of flavone, which is chromone substituted with a phenyl group in the 2-position. In isoflavone, the phenyl group is in the 4-position. Isoflavone is of liminted interest per se, but substituted derivatives are of nutritional interest. Substituted deriv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xenoestrogen
Xenoestrogens are a type of xenohormone that imitates estrogen. They can be either synthetic or natural chemical compounds. Synthetic xenoestrogens include some widely used industrial compounds, such as PCBs, BPA, and phthalates, which have estrogenic effects on a living organism even though they differ chemically from the estrogenic substances produced internally by the endocrine system of any organism. Natural xenoestrogens include phytoestrogens which are plant-derived xenoestrogens. Because the primary route of exposure to these compounds is by consumption of phytoestrogenic plants, they are sometimes called "dietary estrogens". Mycoestrogens, estrogenic substances from fungi, are another type of xenoestrogen that are also considered mycotoxins. Xenoestrogens are clinically significant because they can mimic the effects of endogenous estrogen and thus have been implicated in precocious puberty and other disorders of the reproductive system. Xenoestrogens include pharmacolo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prenylflavonoid
Prenylated flavonoids or prenylflavonoids are a sub-class of flavonoids. They are widely distributed throughout the plant kingdom. Some are known to have phytoestrogenic or antioxidant properties. They are given in the list of adaptogens in herbalism. Chemically they have a prenyl group attached to their flavonoid backbone. It is usually assumed that the addition of hydrophobic prenyl groups facilitate attachment to cell membranes. Prenylation may increase the potential activity of its original flavonoid. Monoprenyl isoflavone epoxidase is a key enzyme in fungal ''Botrytis cinerea'' metabolism of prenylated flavonoids. Many prenylflavonoids appear to have anticancer activity in vitro. Prenylchalcones, prenylflavones, prenylflavonols and prenylflavanones are classes of prenylflavonoids. Examples 6-Prenylnaringenin, 6-geranylnaringenin, 8-prenylnaringenin and isoxanthohumol can be found in hops and beer. Of the prenylflavonoids, 8-prenylnaringenin is the most potent phytoest ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coumestan
Coumestan is a heterocyclic organic compound. Coumestan forms the central core of a variety of natural compounds known collectively as coumestans. Coumestans are oxidation products of pterocarpan that are similar to coumarin. Coumestans, including coumestrol, a phytoestrogen, are found in a variety of plants. Food sources high in coumestans include split peas, pinto beans, lima beans, and especially alfalfa and clover sprouts. Coumestrol has about the same binding affinity for the ER-β estrogen receptor as 17β-estradiol, but much less affinity than 17α-estradiol, although the estrogenic potency of coumestrol at both receptors is much less than that of 17β-estradiol. Because of the estrogenic activity of some coumestans, a variety of syntheses have been developed that allow the preparation of coumestans so that their pharmacological effects can be explored. Coumestans Image:coumestrol.png, Coumestrol Coumestrol is a natural organic compound in the class of phytoche ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Red Clover
''Trifolium pratense'', the red clover, is a herbaceous species of flowering plant in the bean family Fabaceae, native to Europe, Western Asia, and northwest Africa, but planted and naturalized in many other regions. Description Red clover is a herbaceous, short-lived perennial plant, variable in size, growing to tall. It has a deep taproot which makes it tolerant to drought and gives it a good soil structuring effect. The leaves are alternate, trifoliate (with three leaflets), each leaflet long and broad, green with a characteristic pale crescent in the outer half of the leaf; the petiole is long, with two basal stipules that are abruptly narrowed to a bristle-like point. The flowers are dark pink with a paler base, long, produced in a dense inflorescence, and are mostly visited by bumblebees. Distribution The red clover is native to Europe, Western Asia, and northwest Africa, but it has been naturalized in other continents, like North and South America. Specifical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ligand (biochemistry)
In biochemistry and pharmacology, a ligand is a substance that forms a complex with a biomolecule to serve a biological purpose. The etymology stems from ''ligare'', which means 'to bind'. In protein-ligand binding, the ligand is usually a molecule which produces a signal by binding to a site on a target protein. The binding typically results in a change of conformational isomerism (conformation) of the target protein. In DNA-ligand binding studies, the ligand can be a small molecule, ion, or protein which binds to the DNA double helix. The relationship between ligand and binding partner is a function of charge, hydrophobicity, and molecular structure. Binding occurs by intermolecular forces, such as ionic bonds, hydrogen bonds and Van der Waals forces. The association or docking is actually reversible through dissociation. Measurably irreversible covalent bonding between a ligand and target molecule is atypical in biological systems. In contrast to the definition of lig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Estrogen Receptor Beta
Estrogen receptor beta (ERβ) also known as NR3A2 (nuclear receptor subfamily 3, group A, member 2) is one of two main types of estrogen receptor—a nuclear receptor which is activated by the sex hormone estrogen. In humans ERβ is encoded by the ''ESR2'' gene. Function ERβ is a member of the family of estrogen receptors and the superfamily of nuclear receptor transcription factors. The gene product contains an N-terminal DNA binding domain and C-terminal ligand binding domain and is localized to the nucleus, cytoplasm, and mitochondria. Upon binding to 17-β-estradiol, estriol or related ligands, the encoded protein forms homo-dimers or hetero-dimers with estrogen receptor α that interact with specific DNA sequences to activate transcription. Some isoforms dominantly inhibit the activity of other estrogen receptor family members. Several alternatively spliced transcript variants of this gene have been described, but the full-length nature of some of these variants h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Estrogen Receptor Alpha
Estrogen receptor alpha (ERα), also known as NR3A1 (nuclear receptor subfamily 3, group A, member 1), is one of two main types of estrogen receptor, a nuclear receptor (mainly found as a chromatin-binding protein) that is activated by the sex hormone estrogen. In humans, ERα is encoded by the gene ''ESR1'' (EStrogen Receptor 1). Structure The estrogen receptor (ER) is a ligand-activated transcription factor composed of several domains important for hormone binding, DNA binding, and activation of transcription. Alternative splicing results in several ESR1 mRNA transcripts, which differ primarily in their 5-prime untranslated regions. The translated receptors show less variability. Ligands Agonists Non-selective * Endogenous estrogens (e.g., estradiol, estrone, estriol, estetrol) * Natural estrogens (e.g., conjugated equine estrogens) * Synthetic estrogens (e.g., ethinylestradiol, diethylstilbestrol) Selective Agonists of ERα selective over ERβ include: * Propylp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Estrogen Receptor
Estrogen receptors (ERs) are a group of proteins found inside cells. They are receptors that are activated by the hormone estrogen ( 17β-estradiol). Two classes of ER exist: nuclear estrogen receptors (ERα and ERβ), which are members of the nuclear receptor family of intracellular receptors, and membrane estrogen receptors (mERs) (GPER (GPR30), ER-X, and Gq-mER), which are mostly G protein-coupled receptors. This article refers to the former (ER). Once activated by estrogen, the ER is able to translocate into the nucleus and bind to DNA to regulate the activity of different genes (i.e. it is a DNA-binding transcription factor). However, it also has additional functions independent of DNA binding. As hormone receptors for sex steroids (steroid hormone receptors), ERs, androgen receptors (ARs), and progesterone receptors (PRs) are important in sexual maturation and gestation. Proteomics There are two different forms of the estrogen receptor, usually referred to as α a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fusarium
''Fusarium'' is a large genus of filamentous fungi, part of a group often referred to as hyphomycetes, widely distributed in soil and associated with plants. Most species are harmless saprobes, and are relatively abundant members of the soil microbial community. Some species produce mycotoxins in cereal crops that can affect human and animal health if they enter the food chain. The main toxins produced by these ''Fusarium'' species are fumonisins and trichothecenes. Despite most species apparently being harmless (some existing on the skin as commensal members of the skin flora), some ''Fusarium'' species and subspecific groups are among the most important fungal pathogens of plants and animals. The name of ''Fusarium'' comes from Latin ''fusus'', meaning a spindle. Taxonomy The taxonomy of the genus is complex. A number of different schemes have been used, and up to 1,000 species have been identified at times, with approaches varying between wide and narrow concepts of speci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mycoestrogens
Mycoestrogens are xenoestrogens produced by fungi. They are sometimes referred to as mycotoxins. Among important mycoestrogens are zearalenone, zearalenol and zearalanol. Although all of these can be produced by various ''Fusarium'' species, zearalenol and zearalanol may also be produced endogenously in ruminants that have ingested zearalenone. Alpha-zearalanol is also produced semisynthetically, for veterinary use; such use is prohibited in the European Union. Mechanism of action Mycoestrogens act as agonists of the estrogen receptors, ERα and ERβ. Sources Mycoestrogens are produced by various strains of fungi, many of which fall under the genus ''Fusarium''. ''Fusarium'' fungi are filamentous fungi that are found in the soil and are associated with plants and some crops, especially cereals. Zearalenone is mainly produced by ''F. graminearum'' and ''F. culmorum'' strains, which inhabit different areas depending on temperature and humidity. ''F. graminearum'' prefers to inha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |