 |
Phytane
Phytane is the isoprenoid alkane formed when phytol, a constituent of chlorophyll, loses its hydroxyl group. When phytol loses one carbon atom, it yields pristane. Other sources of phytane and pristane have also been proposed than phytol. Pristane and phytane are common constituents in petroleum and have been used as proxies for depositional redox conditions, as well as for correlating oil and its source rock (i.e. elucidating where oil formed). In environmental studies, pristane and phytane are target compounds for investigating oil spills. Chemistry Phytane is a non-polar organic compound that is a clear and colorless liquid at room temperature. It is a head-to-tail linked regular isoprenoid with chemical formula C20H42. Phytane has many structural isomers. Among them, crocetane is a tail-to-tail linked isoprenoid and often co-elutes with phytane during gas chromatography (GC) due to its structural similarity. Phytane also has many stereoisomers because of its three st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
Crocetane
Crocetane, or 2,6,11,15-tetramethylhexadecane, is an isoprenoid hydrocarbon compound. Unlike its isomer phytane, crocetane has a tail-to-tail linked isoprenoid skeleton. Crocetane has been detected in modern sediments and geological records as a biomarker, often associated with anaerobic methane oxidation. Research Crocetane was first studied in the late 1920s and early 1930s for the structural identification of crocetin, which is its polyunsaturated diacid analogue. The infrared spectrum was reported in 1950, the mass spectrum was described in 1968 and the 1H and 13C NMR spectra was obtained in 1990s. In 1994, Liangqiao Bian first reported strong 13C depletion in crocetane from anoxic sediments in the Kattegat. Such low 13C content is thought to originate from microbes harvesting biogenic methane, which is always 13C depleted, as a carbon source. Years later several groups made similar observations in either modern or ancient sediments near methane seeps. Crocetane was found in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
|
Pristane
Pristane is a natural saturated terpenoid alkane obtained primarily from shark liver oil, from which its name is derived (Latin ''pristis'', "shark"). It is also found in the stomach oil of birds in the order Procellariiformes and in mineral oil and some foods. Pristane and phytane are used in the fields of geology and environmental science as biomarkers to characterize origins and evolution of petroleum hydrocarbons and coal. It is a transparent oily liquid that is immiscible with water, but soluble in diethyl ether, benzene, chloroform and carbon tetrachloride. Pristane is known to induce autoimmune diseases in rodents. It is used in research to understand the pathogenesis of rheumatoid arthritis and lupus. It is used as a lubricant, a transformer oil, an immunologic adjuvant, and an anti-corrosion agent, biological marker, plasmocytomas inducer and in production of monoclonal antibodies. Biosynthetically, pristane is derived from phytol Phytol (florasol, phytosol) is an a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
 |
Diterpenoid
Diterpenes are a class of chemical compounds composed of four isoprene units, often with the molecular formula C20H32. They are biosynthesized by plants, animals and fungi via the HMG-CoA reductase pathway, with geranylgeranyl pyrophosphate being a primary intermediate. Diterpenes form the basis for biologically important compounds such as retinol, retinal, and phytol. They are known to be antimicrobial and antiinflammatory. Structures As with most terpenes a huge number of potential structures exists, which may be broadly divided according to the number of rings present. Biosynthesis Diterpenes are derived from the addition of one IPP unit to FPP to form geranylgeranyl-pyrophosphate (GGPP). From GGPP, structural diversity is achieved mainly by two classes of enzymes; the diterpene synthases and cytochromes P450. Several diterpenes are produced by plants and cyanobacteria. GGPP is also the precursor for the synthesis of the phytane by the action of the enzyme geranyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
 |
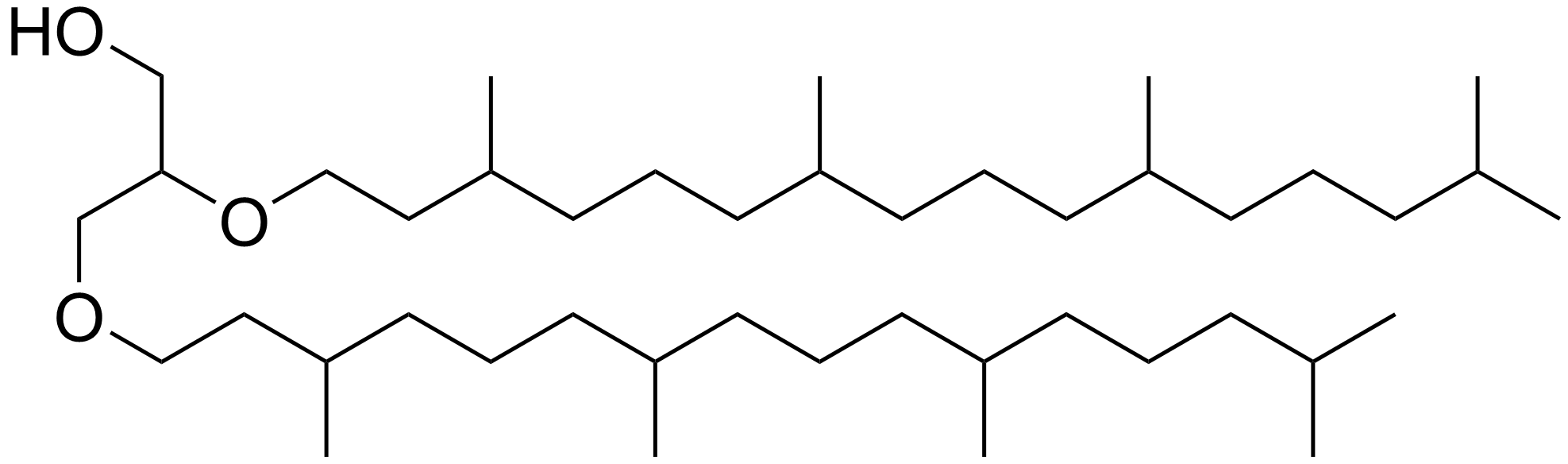
Archaeol
Archaeol is composed of two phytanyl chains linked to the sn-2 and sn-3 positions of glycerol. As its phosphate ester, it is a common component of the membranes of archaea. Structure and contrast with other lipids Archaeol is a diether. The 2,3-sn-glycerol structure and ether bond linkage are two key differences between lipids found in archaea vs those of bacteria and eukarya. The latter use 1,2-sn-glycerol, and mostly, ester bonds. Natural archaeol has 3R, 7R, 11R configurations for the three chiral centers in the isoprenoid chains. There are four structural variations, contributing to the complexity of the membrane lipids in function and properties. The two phytanyl chains can form a 36-member ring to yield macrocyclic archaeol. Hydroxylated archaeol has phytanyl chains hydroxylated at the first tertiary carbon atom, while sesterterpanyl archaeol have the phytanyl side chains with C25 sesterterpanyl chains, substituting at C2 of glycerol or at both carbons. Unsaturated ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
Pristane
Pristane is a natural saturated terpenoid alkane obtained primarily from shark liver oil, from which its name is derived (Latin ''pristis'', "shark"). It is also found in the stomach oil of birds in the order Procellariiformes and in mineral oil and some foods. Pristane and phytane are used in the fields of geology and environmental science as biomarkers to characterize origins and evolution of petroleum hydrocarbons and coal. It is a transparent oily liquid that is immiscible with water, but soluble in diethyl ether, benzene, chloroform and carbon tetrachloride. Pristane is known to induce autoimmune diseases in rodents. It is used in research to understand the pathogenesis of rheumatoid arthritis and lupus. It is used as a lubricant, a transformer oil, an immunologic adjuvant, and an anti-corrosion agent, biological marker, plasmocytomas inducer and in production of monoclonal antibodies. Biosynthetically, pristane is derived from phytol Phytol (florasol, phytosol) is an a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
|
Archaeol
Archaeol is composed of two phytanyl chains linked to the sn-2 and sn-3 positions of glycerol. As its phosphate ester, it is a common component of the membranes of archaea. Structure and contrast with other lipids Archaeol is a diether. The 2,3-sn-glycerol structure and ether bond linkage are two key differences between lipids found in archaea vs those of bacteria and eukarya. The latter use 1,2-sn-glycerol, and mostly, ester bonds. Natural archaeol has 3R, 7R, 11R configurations for the three chiral centers in the isoprenoid chains. There are four structural variations, contributing to the complexity of the membrane lipids in function and properties. The two phytanyl chains can form a 36-member ring to yield macrocyclic archaeol. Hydroxylated archaeol has phytanyl chains hydroxylated at the first tertiary carbon atom, while sesterterpanyl archaeol have the phytanyl side chains with C25 sesterterpanyl chains, substituting at C2 of glycerol or at both carbons. Unsaturated ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
|
List Of Straight-chain Alkanes ...
The following is a list of straight-chain alkanes, the total number of isomers of each (including branched chains), and their common names, sorted by number of carbon atoms. References {{alkanes Alkanes Alkanes In organic chemistry, an alkane, or paraffin (a historical trivial name that also has other meanings), is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in whic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
|
Haloarchaea
Haloarchaea (halophilic archaea, halophilic archaebacteria, halobacteria) are a class of the Euryarchaeota, found in water saturated or nearly saturated with salt. Halobacteria are now recognized as archaea rather than bacteria and are one of the largest groups. The name 'halobacteria' was assigned to this group of organisms before the existence of the domain Archaea was realized, and while valid according to taxonomic rules, should be updated. Halophilic archaea are generally referred to as haloarchaea to distinguish them from halophilic bacteria. These microorganisms are among the halophile organisms, that they require high salt concentrations to grow, with most species requiring more than 2.0M NaCl for growth and survival. They are a distinct evolutionary branch of the Archaea distinguished by the possession of ether-linked lipids and the absence of murein in their cell walls. Haloarchaea can grow aerobically or anaerobically. Parts of the membranes of haloarchaea are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
|
Methanogen
Methanogens are microorganisms that produce methane as a metabolic byproduct in hypoxic conditions. They are prokaryotic and belong to the domain Archaea. All known methanogens are members of the archaeal phylum Euryarchaeota. Methanogens are common in wetlands, where they are responsible for marsh gas, and in the digestive tracts of animals such as ruminants and many humans, where they are responsible for the methane content of belching in ruminants and flatulence in humans. In marine sediments, the biological production of methane, also termed methanogenesis, is generally confined to where sulfates are depleted, below the top layers. Moreover, methanogenic archaea populations play an indispensable role in anaerobic wastewater treatments. Others are extremophiles, found in environments such as hot springs and submarine hydrothermal vents as well as in the "solid" rock of Earth's crust, kilometers below the surface. Physical description Methanogens are coccoid (sph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
|
Substituent
A substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule. (In organic chemistry and biochemistry, the terms ''substituent'' and '' functional group'', as well as '' side chain'' and ''pendant group'', are used almost interchangeably to describe those branches from the parent structure, though certain distinctions are made in polymer chemistry. In polymers, side chains extend from the backbone structure. In proteins, side chains are attached to the alpha carbon atoms of the amino acid backbone.) The suffix ''-yl'' is used when naming organic compounds that contain a single bond replacing one hydrogen; ''-ylidene'' and ''-ylidyne'' are used with double bonds and triple bonds, respectively. In addition, when naming hydrocarbons that contain a substituent, positional numbers are used to indicate which carbon atom the substituent attaches to when such information is needed to distinguish between ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |