|
Photoprotective
Photoprotection is the biochemical process that helps organisms cope with molecular damage caused by sunlight. Plants and other oxygenic phototrophs have developed a suite of photoprotective mechanisms to prevent photoinhibition and oxidative stress caused by excess or fluctuating light conditions. Humans and other animals have also developed photoprotective mechanisms to avoid UV photodamage to the skin, prevent DNA damage, and minimize the downstream effects of oxidative stress. In photosynthetic organisms In organisms that perform oxygenic photosynthesis, excess light may lead to photoinhibition, or photoinactivation of the reaction centers, a process that does not necessarily involve chemical damage. When photosynthetic antenna pigments such as chlorophyll are excited by light absorption, unproductive reactions may occur by charge transfer to molecules with unpaired electrons. Because oxygenic phototrophs generate O2 as a byproduct from the photocatalyzed splitting of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron-starvation-induced Protein A
Iron-starvation-induced protein A, also known as isiA, is a photosynthesis-related chlorophyll-containing protein found in cyanobacteria. It belongs to the chlorophyll-a/b-binding family of proteins, and has been shown to have a photoprotective, photoprotection role in preventing oxidative damage via energy dissipation. It was originally identified under Fe starvation, and thus received the name iron-starvation-induced protein A. However, the protein has more recently been found to respond to a variety of stress conditions such as high irradiance. It can aggregate with carotenoids and form rings around the PSI reaction center complexes to aid in photoprotective energy dissipation. Antenna function IsiA functions as an antenna for photosystem I (PSI) under iron-limiting conditions, when phycobilisomes disappear. In the (PSI)3(Isi3)18 complex most of the harvested energy is probably used by PSI; in other PSI-containing supercomplexes a large part of the energy will probably not be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxybenzone
Oxybenzone or benzophenone-3 or BP-3 (trade names Milestab 9, Eusolex 4360, Escalol 567, KAHSCREEN BZ-3) is an organic compound. It is a pale-yellow solid that is readily soluble in most organic solvents. Oxybenzone belongs to the class of aromatic ketones known as benzophenones. It is widely used in plastics, toys, furniture finishes, and other products to limit UV degradation. Sunscreens containing Oxybenzone have been banned from sale in Hawaii since 2021 due to its environmental effects, such as mortality in developing coral, coral bleaching, genetic damage to coral and marine organisms. NGO's such as EWG, the Environmental Working Group, have petitioned the US FDA to ban the ingredient citing hormonal disruption, cancer related concerns and the FDA's previous ruling in 2021 declaring that it is not GRASE (Generally Recognised As Safe and Effective). Structure and electronic structure Being a conjugated molecule, oxybenzone absorbs light at lower energies than many aroma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sunscreen
Sunscreen, also known as sunblock or sun cream, is a photoprotective topical product for the skin that mainly absorbs, or to a much lesser extent reflects, some of the sun's ultraviolet (UV) radiation and thus helps protect against sunburn and most importantly prevent skin cancer. Sunscreens come as lotions, sprays, gels, foams (such as an expanded foam lotion or whipped lotion), sticks, powders and other topical products. Sunscreens are common supplements to clothing, particularly sunglasses, sunhats and special sun protective clothing, and other forms of photoprotection (such as umbrellas). The first sunscreen in the world was invented in Australia, by chemist H.A. Milton Blake, in 1932 formulating with the UV filter 'salol' (phenyl salicylate) at a concentration of 10%. Its protection was verified by the University of Adelaide and it was also produced commercially by Blake's company, Hamilton Laboratories. Despite sunscreen being relatively new, sun protection practices ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Non-photochemical Quenching
Non-photochemical quenching (NPQ) is a mechanism employed by plants and algae to protect themselves from the adverse effects of high light intensity. It involves the quenching of singlet excited state chlorophylls (Chl) via enhanced internal conversion to the ground state (non-radiative decay), thus harmlessly dissipating excess excitation energy as heat through molecular vibrations. NPQ occurs in almost all photosynthetic eukaryotes (algae and plants), and helps to regulate and protect photosynthesis in environments where light energy absorption exceeds the capacity for light utilization in photosynthesis. Process When a molecule of chlorophyll absorbs light it is promoted from its ground state to its first singlet excited state. The excited state then has three main fates. Either the energy is; 1. passed to another chlorophyll molecule by Förster resonance energy transfer (in this way excitation is gradually passed to the photochemical reaction centers (photosystem I and ph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xanthophyll Cycle
Xanthophylls (originally phylloxanthins) are yellow pigments that occur widely in nature and form one of two major divisions of the carotenoid group; the other division is formed by the carotenes. The name is from Greek (, "yellow") and (, "leaf"), due to their formation of the yellow band seen in early chromatography of leaf pigments. Molecular structure As both are carotenoids, xanthophylls and carotenes are similar in structure, but xanthophylls contain oxygen atoms while carotenes are ''purely hydrocarbons'', which do not contain oxygen. Their content of oxygen causes xanthophylls to be more polar (in molecular structure) than carotenes, and causes their separation from carotenes in many types of chromatography. (Carotenes are usually more orange in color than xanthophylls.) Xanthophylls present their oxygen either as hydroxyl groups and/or as hydrogen atoms substituted by oxygen atoms when acting as a bridge to form epoxides. Occurrence Like other carotenoids, xant ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photoreceptor Protein
Photoreceptor proteins are light-sensitive proteins involved in the sensing and response to light in a variety of organisms. Some examples are rhodopsin in the photoreceptor cells of the vertebrate retina, phytochrome in plants, and bacteriorhodopsin and bacteriophytochromes in some bacteria. They mediate light responses as varied as visual perception, phototropism and phototaxis, as well as responses to light-dark cycles such as circadian rhythm and other photoperiodisms including control of flowering times in plants and mating seasons in animals. Structure Photoreceptor proteins typically consist of a protein attached to a non-protein chromophore (sometimes referred as photopigment, even so photopigment may also refer to the photoreceptor as a whole). The chromophore reacts to light via photoisomerization or photoreduction, thus initiating a change of the receptor protein which triggers a signal transduction cascade. Chromophores found in photoreceptors include retinal (reti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Titanium Oxide
Titanium oxide may refer to: * Titanium dioxide (titanium(IV) oxide), TiO2 * Titanium(II) oxide (titanium monoxide), TiO, a non-stoichiometric oxide * Titanium(III) oxide (dititanium trioxide), Ti2O3 * Ti3O * Ti2O * δ-TiOx (x= 0.68–0.75) * TinO2n−1 where n ranges from 3–9 inclusive, e.g. Ti3O5, Ti4O7, etc. Uses Often used as an active ingredient in sunscreens combined with oxybenzone and octyl methoxycinnamate Octyl methoxycinnamate or ethylhexyl methoxycinnamate (INCI) or octinoxate (USAN), trade names Eusolex 2292 and Uvinul MC80, is an organic compound that is an ingredient in some sunscreens and lip balms. It is an ester formed from methoxycinnam ....Serpone N, Salinaro A, Emeline AV, Horikoshi S, Hidaka H, Zhao JC. 2002. "An in vitro systematic spectroscopic examination of the photostabilities of a random set of commercial sunscreen lotions and their chemical UVB/UVA active agents". ''Photochemical & Photobiological Sciences'' 1(12): 970–981. Used to give the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzophenone
Benzophenone is the organic compound with the formula (C6H5)2CO, generally abbreviated Ph2CO. It is a white solid that is soluble in organic solvents. Benzophenone is a widely used building block in organic chemistry, being the parent diarylketone. Uses Benzophenone can be used as a photo initiator in UV(Ultra-violet)-curing applications such as inks, imaging, and clear coatings in the printing industry. Benzophenone prevents ultraviolet ( UV) light from damaging scents and colors in products such as perfumes and soaps. Benzophenone can also be added to plastic packaging as a UV blocker to prevent photo-degradation of the packaging polymers or its contents. Its use allows manufacturers to package the product in clear glass or plastic (such as a PETE water bottle). Without it, opaque or dark packaging would be required. In biological applications, benzophenones have been used extensively as photophysical probes to identify and map peptide–protein interactions. Benzophenone ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photosensitizers
Photosensitizers produce a physicochemical change in a neighboring molecule by either donating an electron to the substrate or by abstracting a hydrogen atom from the substrate. At the end of this process, the photosensitizer eventually returns to its ground state, where it remains chemically intact until the photosensitizer absorbs more light. This means that the photosensitizer remains unchanged before and after the energetic exchange, much like heterogeneous photocatalysis. One branch of chemistry which frequently utilizes photosensitizers is polymer chemistry, using photosensitizers in reactions such as photopolymerization, photocrosslinking, and photodegradation. Photosensitizers are also used to generate prolonged excited electronic states in organic molecules with uses in photocatalysis, photon upconversion and photodynamic therapy. Generally, photosensitizers absorb electromagnetic radiation consisting of infrared radiation, visible light radiation, and ultraviolet radiati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Free Radicals
In chemistry, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired valence electron. With some exceptions, these unpaired electrons make radicals highly chemically reactive. Many radicals spontaneously dimerize. Most organic radicals have short lifetimes. A notable example of a radical is the hydroxyl radical (HO·), a molecule that has one unpaired electron on the oxygen atom. Two other examples are triplet oxygen and triplet carbene (꞉) which have two unpaired electrons. Radicals may be generated in a number of ways, but typical methods involve redox reactions. Ionizing radiation, heat, electrical discharges, and electrolysis are known to produce radicals. Radicals are intermediates in many chemical reactions, more so than is apparent from the balanced equations. Radicals are important in combustion, atmospheric chemistry, polymerization, plasma chemistry, biochemistry, and many other chemical processes. A majority of n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
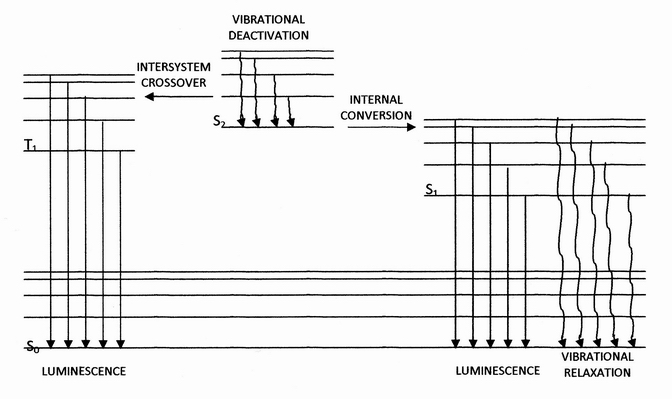
Internal Conversion (chemistry)
Internal conversion is a transition from a higher to a lower electronic state in a molecule or atom.A general and quantitative discussion of intramolecular radiationless transitions is the subject of an article by M. Bixon and J. Jortner (''J. Chem. Phys.'', 48 (2) 715-726 (1968)). It is sometimes called "radiationless de-excitation", because no photons are emitted. It differs from intersystem crossing in that, while both are radiationless methods of de-excitation, the molecular spin state for internal conversion remains the same, whereas it changes for intersystem crossing. The energy of the electronically excited state is given off to vibrational modes of the molecule. The excitation energy is transformed into heat. Examples A classic example of this process is the quinine sulfate fluorescence, which can be quenched by the use of various halide salts. The excited molecule can de-excite by increasing the thermal energy of the surrounding solvated ions. Several natural molecules ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
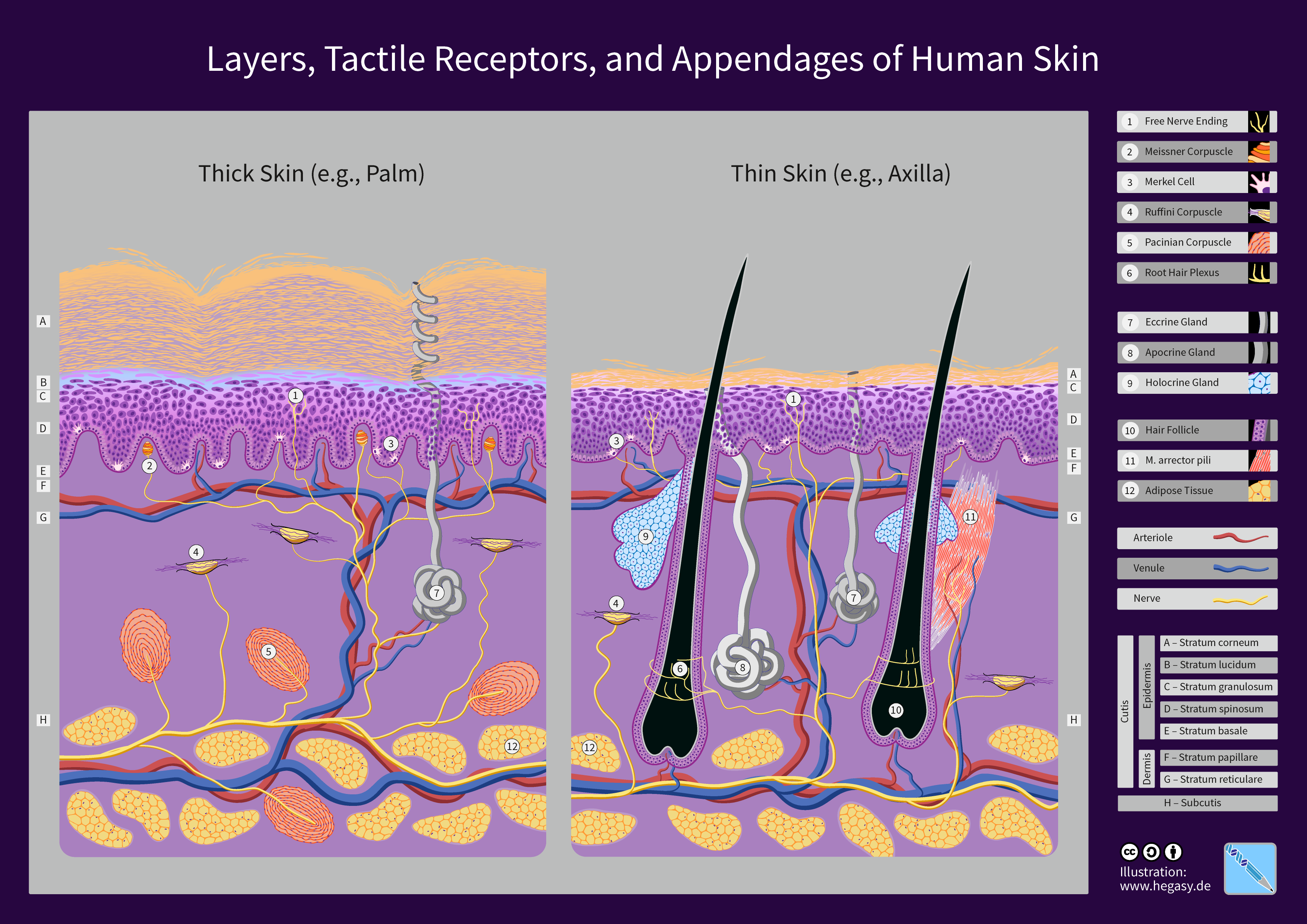
Human Skin
The human skin is the outer covering of the body and is the largest organ of the integumentary system. The skin has up to seven layers of ectodermal tissue guarding muscles, bones, ligaments and internal organs. Human skin is similar to most of the other mammals' skin, and it is very similar to pig skin. Though nearly all human skin is covered with hair follicles, it can appear hairless. There are two general types of skin, hairy and glabrous skin (hairless). The adjective ''cutaneous'' literally means "of the skin" (from Latin ''cutis'', skin). Because it interfaces with the environment, skin plays an important immunity role in protecting the body against pathogens and excessive water loss. Its other functions are insulation, temperature regulation, sensation, synthesis of vitamin D, and the protection of vitamin B folates. Severely damaged skin will try to heal by forming scar tissue. This is often discoloured and depigmented. In humans, skin pigmentation (affected b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |




.jpg)