|
Photographic Fixer
Photographic fixer is a mix of chemicals used in the final step in the photographic processing of film or paper. The fixer stabilises the image, removing the unexposed silver halide remaining on the photographic film or photographic paper, leaving behind the reduced metallic silver that forms the image. By fixation, the film or paper is insensitive to further action by light. Without fixing, the remaining silver halide would darken and cause fogging of the image. Fixation is commonly achieved by treating the film or paper with a solution of thiosulfate salt. Popular salts are sodium thiosulfate—commonly called hypo—and ammonium thiosulfate—commonly used in modern rapid fixer formulae. Fixation involves these chemical reactions (X = halide, typically Br−):Karlheinz Keller et al. "Photography" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. :AgX + 2 S2O32− → g(S2O3)2sup>3− + X− :AgX + 3 S2O32− → g(S2O3)3sup ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photographic Processing
Photographic processing or photographic development is the chemical means by which photographic film or paper is treated after photographic exposure to produce a negative or positive image. Photographic processing transforms the latent image into a visible image, makes this permanent and renders it insensitive to light.Karlheinz Keller et al. "Photography" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. All processes based upon the gelatin silver process are similar, regardless of the film or paper's manufacturer. Exceptional variations include instant films such as those made by Polaroid and thermally developed films. Kodachrome required Kodak's proprietary K-14 process. Kodachrome film production ceased in 2009, and K-14 processing is no longer available as of December 30, 2010. Ilfochrome materials use the dye destruction process. Deliberately using the wrong process for a film is known as cross processing. Common processes All p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silver Halide
A silver halide (or silver salt) is one of the chemical compounds that can form between the Chemical element, element silver (Ag) and one of the halogens. In particular, bromine (Br), chlorine (Cl), iodine (I) and fluorine (F) may each combine with silver to produce silver bromide (AgBr), silver chloride (AgCl), silver iodide (AgI), and three forms of silver fluoride, respectively. As a group, they are often referred to as the silver halides, and are often given the pseudo-chemical notation AgX. Although most silver halides involve silver atoms with oxidation states of +1 (Ag+), silver halides in which the silver atoms have oxidation states of +2 (Ag2+) are known, of which silver(II) fluoride is the only known stable one. Silver halides are light-sensitive chemicals, and are commonly used in photographic film and paper. Applications Light sensitivity Silver halides are used in photographic film and photographic paper, including graphic art film and paper, where silver halide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
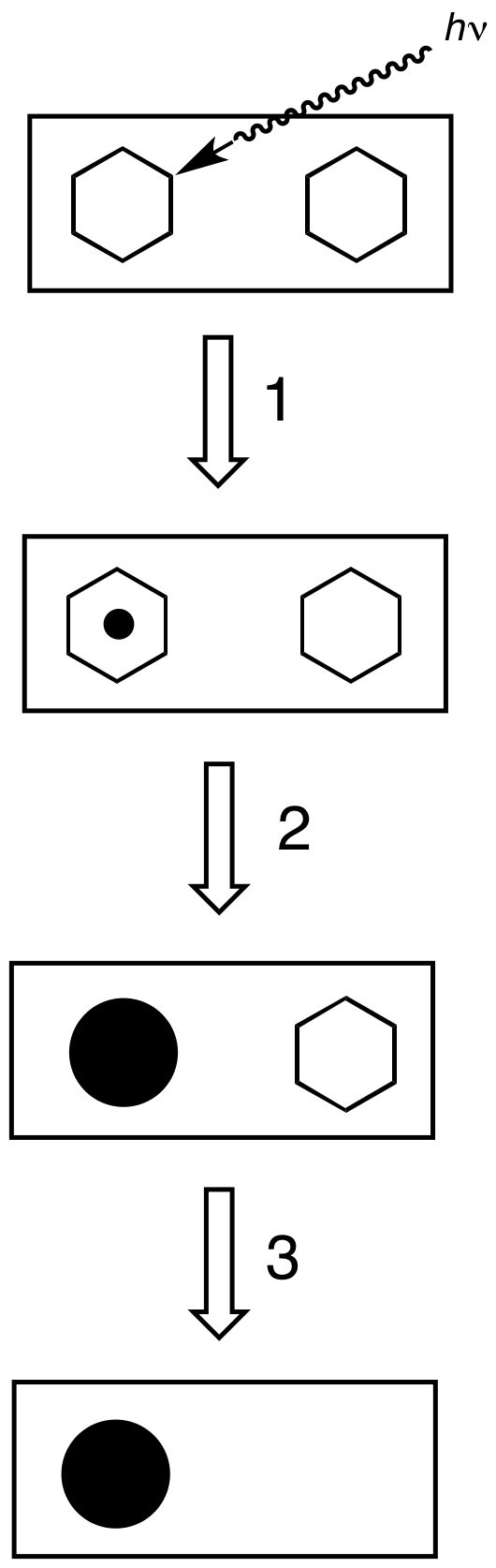
Photographic Film
Photographic film is a strip or sheet of transparent film base coated on one side with a gelatin emulsion containing microscopically small light-sensitive silver halide crystals. The sizes and other characteristics of the crystals determine the sensitivity, contrast, and resolution of the film. The emulsion will gradually darken if left exposed to light, but the process is too slow and incomplete to be of any practical use. Instead, a very short exposure to the image formed by a camera lens is used to produce only a very slight chemical change, proportional to the amount of light absorbed by each crystal. This creates an invisible latent image in the emulsion, which can be chemically developed into a visible photograph. In addition to visible light, all films are sensitive to ultraviolet light, X-rays, gamma rays, and high-energy particles. Unmodified silver halide crystals are sensitive only to the blue part of the visible spectrum, producing unnatural-looking rendit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photographic Paper
Photographic paper is a paper coated with a light-sensitive chemical formula, like photographic film, used for making photographic prints. When photographic paper is exposed to light, it captures a latent image that is then developed to form a visible image; with most papers the image density from exposure can be sufficient to not require further development, aside from fixing and clearing, though latent exposure is also usually present. The light-sensitive layer of the paper is called the emulsion. The most common chemistry was based on silver halide (the focus of this page) but other alternatives have also been used. The print image is traditionally produced by interposing a photographic negative between the light source and the paper, either by direct contact with a large negative (forming a contact print) or by projecting the shadow of the negative onto the paper (producing an enlargement). The initial light exposure is carefully controlled to produce a gray scale image on t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fogging (photography)
Fogging in photography is the deterioration in the quality of the image or the negative caused either by extraneous light, other electromagnetic radiation, radioactivity or the effects of a processing chemical. It is seen either as deposition of silver or dyes across all or part of the image unrelated to the original exposure. It can be confused with chemical staining that can be produced from poorly compounded developer, contamination of processing baths or poor washing after processing. Light Light fogging is where unintended light reaches the photographic material prior to processing is seen as dark areas in the negative which tend to occur over the full width of the film including the margins. This can occur to the film in the camera because of a defect in the manufacture or use of the camera and is seen as dark areas in the negative which tend to occur over the full width of the film including the margins. In 35mm film shadowing from the sprocket holes may be seen on the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Thiosulfate
Sodium thiosulfate (sodium thiosulphate) is an inorganic compound with the formula . Typically it is available as the white or colorless pentahydrate, . The solid is an efflorescent (loses water readily) crystalline substance that dissolves well in water. Sodium thiosulfate is used in gold mining, water treatment, analytical chemistry, the development of silver-based photographic film and prints, and medicine. The medical uses of sodium thiosulfate include treatment of cyanide poisoning and pityriasis. It is on the World Health Organization's List of Essential Medicines. Uses Sodium thiosulfate is used predominantly in industry. For example, it is used to convert dyes to their soluble colorless forms, which are called leuco. It is also used to bleach "wool, cotton, silk, ...soaps, glues, clay, sand, bauxite, and... edible oils, edible fats, and gelatin." Medical uses Sodium thiosulfate is used in the treatment of cyanide poisoning. Other uses include topical treatment ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium Thiosulfate
Ammonium thiosulfate (ammonium thiosulphate in British English) is an inorganic compound with the formula . It is white crystalline solid with ammonia odor, readily soluble in water, slightly soluble in acetone and insoluble in ethanol and diethyl ether. Production It is produced by treating ammonium sulfite with sulfur at temperatures between 85 and 110 °C: : Applications Ammonium thiosulfate is used in photographic fixer. It is a so-called rapid fixer, acting more quickly than sodium thiosulfate fixers. Fixation involves these chemical reactions (illustrated for silver bromide): : : Ammonium thiosulfate is also used for leaching of gold and silver. It works with presence of copper as a catalyst here. This process is a nontoxic alternative gold cyanidation. The advantage to ammonium thiosulfate is that the pyrolysis of its silver complexes leaves a residue solely of silver sulfide, in contrast to complexes derived from sodium thiosulfate. Other Ammonium thiosulfate can be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fluoride, chloride, bromide, iodide, astatide, or theoretically tennesside compound. The alkali metals combine directly with halogens under appropriate conditions forming halides of the general formula, MX (X = F, Cl, Br or I). Many salts are halides; the ''hal-'' syllable in ''halide'' and ''halite'' reflects this correlation. All Group 1 metals form halides that are white solids at room temperature. A halide ion is a halogen atom bearing a negative charge. The halide anions are fluoride (), chloride (), bromide (), iodide () and astatide (). Such ions are present in all ionic halide salts. Halide minerals contain halides. All these halides are colourless, high melting crystalline solids having high negative enthalpies of form ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photographic Developer
In the processing of photographic films, plates or papers, the photographic developer (or just developer) is one or more chemicals that convert the latent image to a visible image. Developing agents achieve this conversion by reducing the silver halides, which are pale-colored, into silver metal, which is black (when a fine particle).Karlheinz Keller et al. ''Photography'' in ''Ullmann's Encyclopedia of Industrial Chemistry'', 2005, Wiley-VCH, Weinheim. The conversion occurs within the gelatine matrix. The special feature of photography is that the developer acts more quickly on those particles of silver halides that have been exposed to light. Paper left in developer will eventually reduce all the silver halides and turn black. Generally, the longer a developer is allowed to work, the darker the image. Chemical composition of developers The developer typically consists of a mixture of chemical compounds prepared as an aqueous solution. For black-and-white photography, three ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bisulfite
The bisulfite ion (IUPAC-recommended nomenclature: hydrogensulfite) is the ion . Salts containing the ion are also known as "sulfite lyes". Sodium bisulfite is used interchangeably with sodium metabisulfite (Na2S2O5). Sodium metabisulfite dissolves in water to give a solution of Na+. :Na2S2O5 + H2O → 2Na SO3 Structure The bisulfite anion exists in solution as a mixture of two tautomers. One tautomer has the proton attached to one of the three oxygen centers. In the second tautomer the proton resides on sulfur. The S-protonated tautomer has ''C''3v symmetry. The O-protonated tautomer has only Cs symmetry. Reactions Tautomerization There exist two tautomers of bisulfite. They interconvert readily but can be characterized individually by various spectroscopic methods. They have been observed by 17O NMR spectroscopy: :HSO3− SO2(OH)− K = 4.2 Acid-base reactions Solutions of bisulfite are typically prepared by treatment of sulfur dioxide with aqueous base: : ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kodachrome
Kodachrome is the brand name for a color reversal film introduced by Eastman Kodak in 1935. It was one of the first successful color materials and was used for both cinematography and still photography. For many years Kodachrome was widely used for professional color photography, especially for images intended for publication in print media. Because of its complex processing requirements, the film was initially exclusively sold process-paid in the United States: customers had to pay Kodak for the cost of development when they bought the film, and independent photography stores were prohibited from developing Kodachrome photos. To develop the film, customers had to mail film to Kodak, who mailed the developed photos back for no additional charge. In 1954, the U.S. Department of Justice found this practice to be an uncompetitive violation of antitrust law. Kodak entered into a consent decree requiring they offer Kodachrome film for sale with and without the development fee, as wel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromogenic
In chemistry, the term chromogen refers to a colourless (or faintly coloured) chemical compound that can be converted by chemical reaction into a compound which can be described as "coloured". There is no universally agreed definition of the term. Various dictionaries give the following definitions: * A substance capable of conversion into a pigment or dye. * Any substance that can become a pigment or coloring matter, a substance in organic fluids that forms colored compounds when oxidized, or a compound, not itself a dye, that can become a dye. * Any substance, itself without color, giving origin to a coloring matter. In biochemistry the term has a rather different meaning. The following are found in various dictionaries. * A precursor of a biochemical pigment * A pigment-producing microorganism * Any of certain bacteria that produce a pigment * A strongly pigmented or pigment-generating organelle, organ, or microorganism. Applications in chemistry *In chromogenic photography, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |