|
Phosphoribosyl-N-formylglycineamide
Phosphoribosyl-''N''-formylglycineamide (or FormylGlycinAmideRibotide, FGAR) is a biochemical intermediate in the formation of purine nucleotides via inosine-5-monophosphate, and hence is a building block for DNA and RNA. The vitamins thiamine and cobalamin also contain fragments derived from FGAR. FGAR is formed when the enzyme phosphoribosylglycinamide formyltransferase adds a formyl group from 10-formyltetrahydrofolate to glycineamide ribonucleotide (GAR) in reaction : :GAR + 10-formyltetrahydrofolate → FGAR + tetrahydrofolate The biosynthesis pathway next converts FGAR to an amidine by the action of phosphoribosylformylglycinamidine synthase (), transferring an amino group from glutamine and giving 5'-phosphoribosylformylglycinamidine (FGAM) in a reaction that also requires ATP: :FGAR + ATP + glutamine + H2O → FGAM + ADP + glutamate + Pi See also * 5-Aminoimidazole ribotide * Purine metabolism Purine metabolism refers to the metabolic pathways to synthesize and bre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphoribosylglycinamide Formyltransferase
Phosphoribosylglycinamide formyltransferase (, ''2-amino-N-ribosylacetamide 5'-phosphate transformylase'', ''GAR formyltransferase'', ''GAR transformylase'', ''glycinamide ribonucleotide transformylase'', ''GAR TFase'', ''5,10-methenyltetrahydrofolate:2-amino-N-ribosylacetamide ribonucleotide transformylase'') is an enzyme with systematic name ''10-formyltetrahydrofolate:5'-phosphoribosylglycinamide N-formyltransferase''. This enzyme catalyses the following chemical reaction : 10-formyltetrahydrofolate + N1-(5-phospho-D-ribosyl)glycinamide \rightleftharpoons tetrahydrofolate + N2-formyl-N1-(5-phospho-D-ribosyl)glycinamide This THF dependent enzyme catalyzes a nucleophilic acyl substitution of the formyl group from 10-formyltetrahydrofolate (fTHF) to N1-(5-phospho-D-ribosyl)glycinamide (GAR) to form N2-formyl-N1-(5-phospho-D-ribosyl)glycinamide (fGAR) as shown above. This reaction plays an important role in the formation of purine through the ''de novo'' purine biosynthesis pa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycineamide Ribonucleotide
Glycineamide ribonucleotide (or GAR) is a biochemical intermediate in the formation of purine nucleotides via inosine-5-monophosphate, and hence is a building block for DNA and RNA. The vitamins thiamine and cobalamin also contain fragments derived from GAR. : GAR is the product of the enzyme phosphoribosylamine—glycine ligase acting on phosphoribosylamine (PRA) to combine it with glycine in a process driven by ATP. The reaction, forms an amide bond: : + + ATP → + ADP + Pi The biosynthesis pathway next adds a formyl group from 10-formyltetrahydrofolate to GAR, catalysed by phosphoribosylglycinamide formyltransferase in reaction and producing formylglycinamide ribotide (FGAR): :GAR + 10-formyltetrahydrofolate → FGAR + tetrahydrofolate See also * 5-Aminoimidazole ribotide * Purine metabolism Purine metabolism refers to the metabolic pathways to synthesize and break down purines that are present in many organisms. Biosynthesis Purines are biologically synthesi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Purine Metabolism
Purine metabolism refers to the metabolic pathways to synthesize and break down purines that are present in many organisms. Biosynthesis Purines are biologically synthesized as nucleotides and in particular as ribotides, i.e. bases attached to ribose 5-phosphate. Both adenine and guanine are derived from the nucleotide inosine monophosphate (IMP), which is the first compound in the pathway to have a completely formed purine ring system. IMP Inosine monophosphate is synthesized on a pre-existing ribose-phosphate through a complex pathway (as shown in the figure on the right). The source of the carbon and nitrogen atoms of the purine ring, 5 and 4 respectively, come from multiple sources. The amino acid glycine contributes all its carbon (2) and nitrogen (1) atoms, with additional nitrogen atoms from glutamine (2) and aspartic acid (1), and additional carbon atoms from formyl groups (2), which are transferred from the coenzyme tetrahydrofolate as 10-formyltetrahydrofolate, and a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
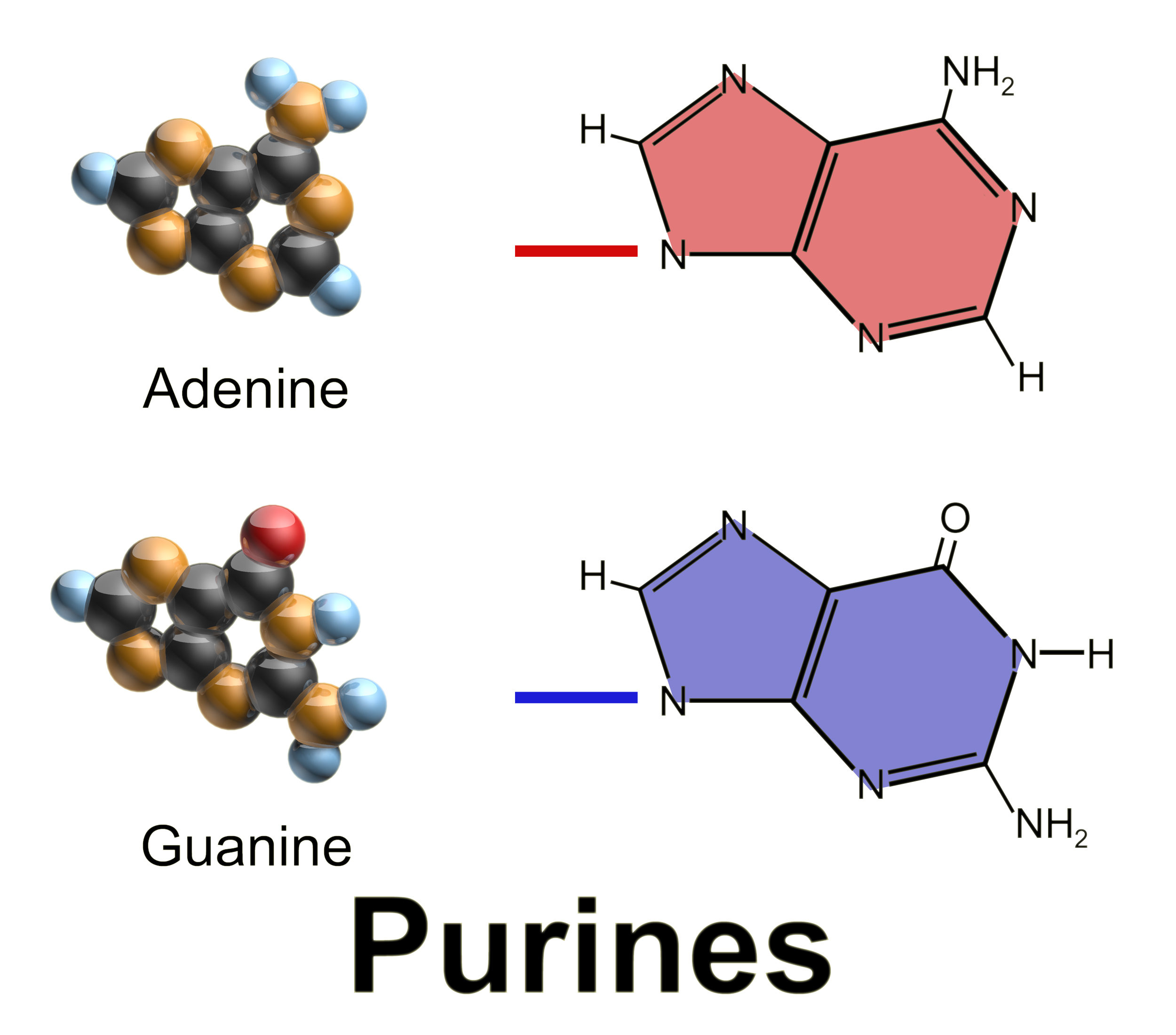
Purine
Purine is a heterocyclic compound, heterocyclic aromatic organic compound that consists of two rings (pyrimidine and imidazole) fused together. It is water-soluble. Purine also gives its name to the wider class of molecules, purines, which include substituted purines and their tautomers. They are the most widely occurring nitrogen-containing heterocycles in nature. Dietary sources Purines are found in high concentration in meat and meat products, especially internal organs such as liver and kidney. In general, plant-based diets are low in purines. High-purine plants and algae include some legumes (lentils and Black-eyed pea, black eye peas) and Spirulina (dietary supplement), spirulina. Examples of high-purine sources include: sweetbreads, Anchovies as food, anchovies, Sardines as food, sardines, liver, beef kidneys, Brain as food, brains, meat extracts (e.g., Oxo (food), Oxo, Bovril), herring, mackerel, scallops, game meats, yeast (beer, yeast extract, nutritional yeast) and g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleotides
Nucleotides are organic molecules consisting of a nucleoside and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both of which are essential biomolecules within all life-forms on Earth. Nucleotides are obtained in the diet and are also synthesized from common nutrients by the liver. Nucleotides are composed of three subunit molecules: a nucleobase, a five-carbon sugar (ribose or deoxyribose), and a phosphate group consisting of one to three phosphates. The four nucleobases in DNA are guanine, adenine, cytosine and thymine; in RNA, uracil is used in place of thymine. Nucleotides also play a central role in metabolism at a fundamental, cellular level. They provide chemical energy—in the form of the nucleoside triphosphates, adenosine triphosphate (ATP), guanosine triphosphate (GTP), cytidine triphosphate (CTP) and uridine triphosphate (UTP)—throughout the cell for the many cellular fun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inosine
Inosine is a nucleoside that is formed when hypoxanthine is attached to a ribose ring (also known as a ribofuranose) via a β-N9-glycosidic bond. It was discovered in 1965 in analysis of RNA transferase. Inosine is commonly found in tRNAs and is essential for proper translation of the genetic code in wobble base pairs. Knowledge of inosine metabolism has led to advances in immunotherapy in recent decades. Inosine monophosphate is oxidised by the enzyme inosine monophosphate dehydrogenase, yielding xanthosine monophosphate, a key precursor in purine metabolism. Mycophenolate mofetil is an anti-metabolite, anti-proliferative drug that acts as an inhibitor of inosine monophosphate dehydrogenase. It is used in the treatment of a variety of autoimmune diseases including granulomatosis with polyangiitis because the uptake of purine by actively dividing B cells can exceed 8 times that of normal body cells, and, therefore, this set of white cells (which cannot operate purine salvage p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiamine
Thiamine, also known as thiamin and vitamin B1, is a vitamin, an essential micronutrient, that cannot be made in the body. It is found in food and commercially synthesized to be a dietary supplement or medication. Phosphorylated forms of thiamine are required for some metabolic reactions, including the breakdown of glucose and amino acids. Food sources of thiamine include whole grains, legumes, and some meats and fish. Grain processing removes much of the vitamin content, so in many countries cereals and flours are enriched with thiamine. Supplements and medications are available to treat and prevent thiamine deficiency and disorders that result from it include beriberi and Wernicke encephalopathy. They are also used to treat maple syrup urine disease and Leigh syndrome. Supplements and medications are typically taken by mouth, but may also be given by intravenous or intramuscular injection. Thiamine supplements are generally well tolerated. Allergic reactions, including ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cobalamin
Vitamin B12, also known as cobalamin, is a water-soluble vitamin involved in metabolism. It is one of eight B vitamins. It is required by animals, which use it as a cofactor in DNA synthesis, in both fatty acid and amino acid metabolism. It is important in the normal functioning of the nervous system via its role in the synthesis of myelin, and in the circulatory system in the maturation of red blood cells in the bone marrow. Plants do not need cobalamin and carry out the reactions with enzymes that are not dependent on it. Vitamin B12 is the most chemically complex of all vitamins, and for humans, the only vitamin that must be sourced from animal-derived foods or from supplements. Only some archaea and bacteria can synthesize vitamin B12. Most people in developed countries get enough B12 from the consumption of meat or foods with animal sources. Foods containing vitamin B12 include meat, clams, liver, fish, poultry, eggs, and dairy products. Many breakfast cereals are for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amidine
Amidines are organic compounds with the functional group RC(NR)NR2, where the R groups can be the same or different. They are the imine derivatives of amides (RC(O)NR2). The simplest amidine is formamidine, HC(=NH)NH2. Examples of amidines include: * DBU * diminazene * benzamidine * Pentamidine * Paranyline Preparation A common route to primary amidines is the Pinner reaction. Reaction of the nitrile with alcohol in the presence of acid gives an iminoether. Treatment of the resulting compound with ammonia then completes the conversion to the amidine. Instead of using a Bronsted acid, Lewis acids such as aluminium trichloride promote the direct amination of nitriles. They are also generated by amination of an imidoyl chloride. They are also prepared by the addition of organolithium reagents to diimines, followed by protonation or alkylation. Dimethylformamide acetal reacts with primary amines to give amidines: :Me2NC(H)(OMe)2 + RNH2 → Me2NC=NHR + 2 MeOH Properties and appl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphoribosylformylglycinamidine Synthase
In enzymology, a phosphoribosylformylglycinamidine synthase () is an enzyme that catalyzes the chemical reaction :ATP + ''N''2-formyl-''N''1-(5-phospho-D-ribosyl)glycinamide + L-glutamine + H2O \rightleftharpoons ADP + phosphate + 2-(formamido)-''N''1-(5-phospho-D-ribosyl)acetamidine + L-glutamate The 4 substrates of this enzyme are ATP, N2-formyl-N1-(5-phospho-D-ribosyl)glycinamide, L-glutamine, and H2O, whereas its 4 products are ADP, phosphate, 2-(formamido)-N1-(5-phospho-D-ribosyl)acetamidine, and L-glutamate. This enzyme belongs to the family of ligases, specifically those forming carbon-nitrogen bonds carbon-nitrogen ligases with glutamine as amido-N-donor. The systematic name of this enzyme class is N2-formyl-N1-(5-phospho-D-ribosyl)glycinamide:L-glutamine amido-ligase (ADP-forming). Other names in common use include phosphoribosylformylglycinamidine synthetase, formylglycinamide ribonucloetide amidotransferase, phosphoribosylformylglycineamidine synthetase, FGAM syn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |