|
Phosphaalkene
Phosphaalkenes (IUPAC name: alkylidenephosphanes) are organophosphorus compounds with double bonds between carbon and phosphorus(III) with the formula R2C=PR. In the compound phosphorine one carbon atom in benzene is replaced by phosphorus. The reactivity of phosphaalkenes is often compared to that of alkenes and not to that of imines because the HOMO of phosphaalkenes is not the phosphorus lone pair (as in imines the amine lone pair) but the double bond. Therefore like alkenes, phosphaalkenes engage in Wittig reactions, Peterson reactions, Cope rearrangements and Diels-Alder reactions. The first phosphaalkene discovered was a phosphabenzene, by Mërkl in 1969. The first localized phosphaalkene was reported in 1976 by Gerd Becker as a keto-enol tautomerism akin a Brook rearrangement: :: In the same year Harold Kroto established spectroscopically that thermolysis of Me2PH generates CH2=PMe. A general method for the synthesis of phosphaalkenes is by 1,2-elimination of suitab ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organophosphorus Compound
Organophosphorus compounds are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment. Some organophosphorus compounds are highly effective insecticides, although some are extremely toxic to humans, including sarin and VX nerve agents. Organophosphorus chemistry is the corresponding science of the properties and reactivity of organophosphorus compounds. Phosphorus, like nitrogen, is in group 15 of the periodic table, and thus phosphorus compounds and nitrogen compounds have many similar properties. The definition of organophosphorus compounds is variable, which can lead to confusion. In industrial and environmental chemistry, an organophosphorus compound need contain only an organic substituent, but need not have a direct phosphorus-carbon (P-C) bond. Thus a large proportion of pesticides (e.g., malathion), are often included in this class of compounds. Phosphorus can adopt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stable Phosphorus Radicals
Stable and persistent phosphorus radicals are phosphorus-centred radicals that are isolable and can exist for at least short periods of time. Radicals consisting of main group elements are often very reactive and undergo uncontrollable reactions, notably dimerization and polymerization. The common strategies for stabilising these phosphorus radicals usually include the delocalisation of the unpaired electron over a pi system or nearby electronegative atoms, and kinetic stabilisation with bulky ligands. Stable and persistent phosphorus radicals can be classified into three categories: neutral, cationic, and anionic radicals. Each of these classes involve various sub-classes, with neutral phosphorus radicals being the most extensively studied. Phosphorus exists as one isotope 31P (''I'' = 1/2) with large hyperfine couplings relative to other spin active nuclei, making phosphorus radicals particularly attractive for spin-labelling experiments. Neutral phosphorus radicals Neutra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Brook Rearrangement
In organic chemistry the Brook rearrangement refers to any [1,''n''] carbon to oxygen silyl migration. The Rearrangement reaction, rearrangement was first observed in the late 1950s by Canadian chemist Adrian Gibbs Brook (1924–2013), after which the reaction is named. These migrations can be promoted in a number of different ways, including thermally, photolytically or under basic/acidic conditions. In the forward direction, these silyl migrations produce silyl ethers as products which is driven by the stability of the oxygen-silicon bond. The silyl substituents can be Aliphatic compound, aliphatic or Aromatic compound, aromatic, and if the silicon is a center of Chirality (chemistry), chirality, the migration occurs with retention at this center. This migration occurs through a transition state where silicon is penta-Coordination complex, coordinate and bears a partial negative charge. If a center of chirality is present at the carbon center to which the silyl group is attached ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Poly(p-phenylenephosphaalkene)
Poly, from the Greek πολύς meaning "many" or "much", may refer to: Businesses * China Poly Group Corporation, a Chinese business group, and its subsidiaries: ** Poly Property, a Hong Kong incorporated Chinese property developer ** Poly Real Estate, a Chinese real estate developer ** Poly Technologies, a defense manufacturing company * Poly (company), formerly Polycom, an American communications technology company People * Poly (footballer) (1906-1986), full name Policarpo Ribeiro de Oliveira, Brazilian footballer * Natasha Poly (born 1985), stage name of Russian supermodel Natalya Sergeyevna Polevshchikova * Poly Styrene (1957–2011), stage name of British musician Marianne Joan Elliott-Said Other uses * Hong Kong Polytechnic University, locally known as Poly * Poly (website), a website by Google * Polynesian, often shortened to poly, as in ‘Poly people are also called “Pasifika” or “Tangata Moana” (people of the ocean)’ * Polyamory, often shortened to poly, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Poly(p-phenylene Vinylene)
Poly(''p''-phenylene vinylene) (PPV, or polyphenylene vinylene) is a conducting polymer of the rigid-rod polymer family. PPV is the only polymer of this type that can be processed into a highly ordered crystalline thin film. PPV and its derivatives are electrically conducting upon doping. Although insoluble in water, its precursors can be manipulated in aqueous solution. The small optical band gap and its bright yellow fluorescence makes PPV a candidate in applications such as light-emitting diodes (LED) and photovoltaic devices. Moreover, PPV can be doped to form electrically conductive materials. Its physical and electronic properties can be altered by the inclusion of functional side groups. Preparation PPVs can be synthesized by a variety of methods, the details of which determine purity and molecular weight. The most popular methods proceed via p- xylylene intermediates after a base induced elimination from α,α'-disubstituted para-xylenes. : Other methods Although xyly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triethylamine
Triethylamine is the chemical compound with the formula N(CH2CH3)3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine or tetraethylammonium, for which TEA is also a common abbreviation. It is a colourless volatile liquid with a strong fishy odor reminiscent of ammonia. Like diisopropylethylamine (Hünig's base), triethylamine is commonly employed in organic synthesis, usually as a base. Synthesis and properties Triethylamine is prepared by the alkylation of ammonia with ethanol: :NH3 + 3 C2H5OH → N(C2H5)3 + 3 H2O The pKa of protonated triethylamine is 10.75,David Evans Research Group and it can be used to prepare buffer solutions at that pH. The |
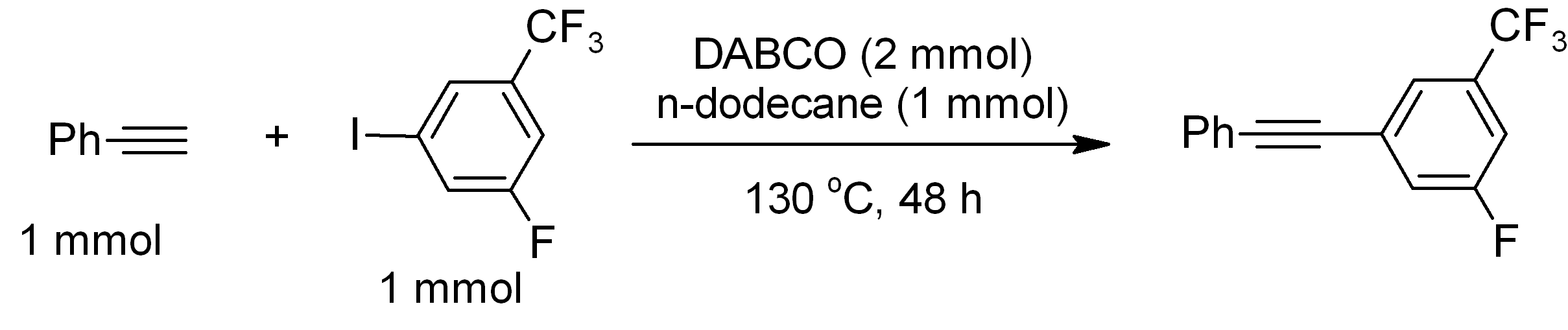
DABCO
DABCO (1,4-diazabicyclo .2.2ctane), also known as triethylenediamine or TEDA, is a bicyclic organic compound with the formula N2(C2H4)3. This colorless solid is a highly nucleophilic tertiary amine base, which is used as a catalyst and reagent in polymerization and organic synthesis. It is similar in structure to quinuclidine, but the latter has one of the nitrogen atoms replaced by a carbon atom. Reactions The p''K''a of DABCOsup>+ (the protonated derivative) is 8.8, which is almost the same as ordinary alkylamines. The nucleophilicity of the amine is high because the amine centers are unhindered. It is sufficiently basic to promote C–C coupling of terminal acetylenes, for example, phenylacetylene couples with electron-deficient iodoarenes. : Catalyst DABCO is used as a base-catalyst for: *formation of polyurethane from alcohol and isocyanate functionalized monomers and pre-polymers. * Baylis-Hillman reactions of aldehydes and unsaturated ketones and aldehydes. : Lewis ba ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DBU (chemistry)
1,8-Diazabicyclo .4.0ndec-7-ene, or more commonly DBU, is a chemical compound and belongs to the class of amidine compounds. It is used in organic synthesis as a catalyst, a complexing ligand, and a non-nucleophilic base. Occurrence Although all commercially available DBU is produced synthetically, it may also be isolated from the sea sponge '' Niphates digitalis''. The biosynthesis of DBU has been proposed to begin with 1,6-hexanedial and 1,3-diaminopropane. Uses As a reagent in organic chemistry, DBU is used as a catalyst, a complexing ligand, and a non-nucleophilic base. It is also used as a curing agent for epoxy resins. It is used in the separation of fullerenes in conjunction with trimethylbenzene. It reacts with C70 and higher fullerenes, but not with to C60 It is also used as a catalyst in the production of polyurethanes. It also exhibited its dual character (base and nucleophile) in the synthesis of aryl- and styryl-terminal acetylenes. See also * 1,5-Diazabic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Elimination Reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one- or two-step mechanism. The one-step mechanism is known as the E2 reaction, and the two-step mechanism is known as the E1 reaction. The numbers refer not to the number of steps in the mechanism, but rather to the kinetics of the reaction: E2 is bimolecular (second-order) while E1 is unimolecular (first-order). In cases where the molecule is able to stabilize an anion but possesses a poor leaving group, a third type of reaction, E1CB, exists. Finally, the pyrolysis of xanthate and acetate esters proceed through an "internal" elimination mechanism, the Ei mechanism. E2 mechanism The E2 mechanism, where E2 stands for bimolecular elimination, involves a one-step mechanism in which ''carbon-hydrogen'' and ''carbon-halogen'' bonds break to form a double bond (''C=C Pi bond''). The specifics of the reaction are as follows: * E2 is a single step elimination, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |