|
Pepsin
Pepsin is an endopeptidase that breaks down proteins into smaller peptides. It is produced in the gastric chief cells of the stomach lining and is one of the main digestive enzymes in the digestive systems of humans and many other animals, where it helps digest the proteins in food. Pepsin is an aspartic protease, using a catalytic aspartate in its active site. It is one of three principal endopeptidases (enzymes cutting proteins in the middle) in the human digestive system, the other two being chymotrypsin and trypsin. There are also exopeptidases which remove individual amino acids at both ends of proteins (carboxypeptidases produced by the pancreas and aminopeptidases secreted by the small intestine). During the process of digestion, these enzymes, each of which is specialized in severing links between particular types of amino acids, collaborate to break down dietary proteins into their components, i.e., peptides and amino acids, which can be readily absorbed by the sma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Theodor Schwann
Theodor Schwann (; 7 December 181011 January 1882) was a German physician and physiologist. His most significant contribution to biology is considered to be the extension of cell theory to animals. Other contributions include the discovery of Schwann cells in the peripheral nervous system, the discovery and study of pepsin, the discovery of the organic nature of yeast, and the invention of the term "metabolism". Early life and education Theodor Schwann was born in Neuss on 7 December 1810 to Leonard Schwann and Elisabeth Rottels. Leonard Schwann was a goldsmith and later a printer. Theodor Schwann studied at the Dreikönigsgymnasium (also known as the Tricoronatum or Three Kings School), a Jesuit school in Cologne. Schwann was a devout Roman Catholic. In Cologne his religious instructor , a priest and novelist, emphasized the individuality of the human soul and the importance of free will. In 1829, Schwann enrolled at the University of Bonn in the premedical curriculum. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Digestive Enzyme
Digestive enzymes are a group of enzymes that break down polymeric macromolecules into their smaller building blocks, in order to facilitate their absorption into the cells of the body. Digestive enzymes are found in the digestive tracts of animals (including humans) and in the tracts of carnivorous plants, where they aid in the digestion of food, as well as inside cells, especially in their lysosomes, where they function to maintain cellular survival. Digestive enzymes of diverse specificities are found in the saliva secreted by the salivary glands, in the secretions of cells lining the stomach, in the pancreatic juice secreted by pancreatic exocrine cells, and in the secretions of cells lining the small and large intestines. Digestive enzymes are classified based on their target substrates: * Lipases split fatty acids into fats and oils. *Proteases and peptidases split proteins into small peptides and amino acids. *Amylases split carbohydrates such as starch and sugars into s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aspartic Protease
Aspartic proteases are a catalytic type of protease enzymes that use an activated water molecule bound to one or more aspartate residues for catalysis of their peptide substrates. In general, they have two highly conserved aspartates in the active site and are optimally active at acidic pH. Nearly all known aspartyl proteases are inhibited by pepstatin. Aspartic endopeptidases of vertebrate, fungal and retroviral origin have been characterised. More recently, aspartic endopeptidases associated with the processing of bacterial type 4 prepilin and archaean preflagellin have been described. Eukaryotic aspartic proteases include pepsins, cathepsins, and renins. They have a two-domain structure, arising from ancestral duplication. Retroviral and retrotransposon proteases (retroviral aspartyl proteases) are much smaller and appear to be homologous to a single domain of the eukaryotic aspartyl proteases. Each domain contributes a catalytic Asp residue, with an extended active site cle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
John Howard Northrop
John Howard Northrop (July 5, 1891 – May 27, 1987) was an American biochemist who, with James Batcheller Sumner and Wendell Meredith Stanley, won the 1946 Nobel Prize in Chemistry. The award was given for these scientists' isolation, crystallization, and study of enzymes, proteins, and viruses. Northrop was a Professor of Bacteriology and Medical Physics, Emeritus, at University of California, Berkeley. Biography Early years Northrop was born in Yonkers, New York to John Isaiah, a zoologist and instructor at Columbia University who is a member of the Havemeyer family, and Alice Rich Northrop, a teacher of botany at Hunter College. His father died in a lab explosion two weeks before John H. Northrop was born. The son was educated at Yonkers High School and Columbia University, where he earned his BA in 1912 and PhD in chemistry in 1915. During World War I, he conducted research for the U.S. Chemical Warfare Service on the production of acetone and ethanol through ferme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zymogen
In biochemistry, a zymogen (), also called a proenzyme (), is an inactive precursor of an enzyme. A zymogen requires a biochemical change (such as a hydrolysis reaction revealing the active site, or changing the configuration to reveal the active site) for it to become an active enzyme. The biochemical change usually occurs in Golgi bodies, where a specific part of the precursor enzyme is cleaved in order to activate it. The inactivating piece which is cleaved off can be a peptide unit, or can be independently-folding domains comprising more than 100 residues. Although they limit the enzyme's ability, these N-terminal extensions of the enzyme or a “prosegment” often aid in the stabilization and folding of the enzyme they inhibit. The pancreas secretes zymogens partly to prevent the enzymes from digesting proteins in the cells in which they are synthesised. Enzymes like pepsin are created in the form of pepsinogen, an inactive zymogen. Pepsinogen is activated when chief ce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Endopeptidase
Endopeptidase or endoproteinase are proteolytic peptidases that break peptide bonds of nonterminal amino acids (i.e. within the molecule), in contrast to exopeptidases, which break peptide bonds from end-pieces of terminal amino acids. For this reason, endopeptidases cannot break down peptides into monomers, while exopeptidases can break down proteins into monomers. A particular case of endopeptidase is the oligopeptidase, whose substrates are oligopeptides instead of proteins. They are usually very specific for certain amino acids. Examples of endopeptidases include: * Trypsin - cuts after Arg or Lys, unless followed by Pro. Very strict. Works best at pH 8. * Chymotrypsin - cuts after Phe, Trp, or Tyr, unless followed by Pro. Cuts more slowly after His, Met or Leu. Works best at pH 8. * Elastase - cuts after Ala, Gly, Ser, or Val, unless followed by Pro. * Thermolysin - cuts ''before'' Ile, Met, Phe, Trp, Tyr, or Val, unless ''preceded'' by Pro. Sometimes cuts after Ala, Asp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
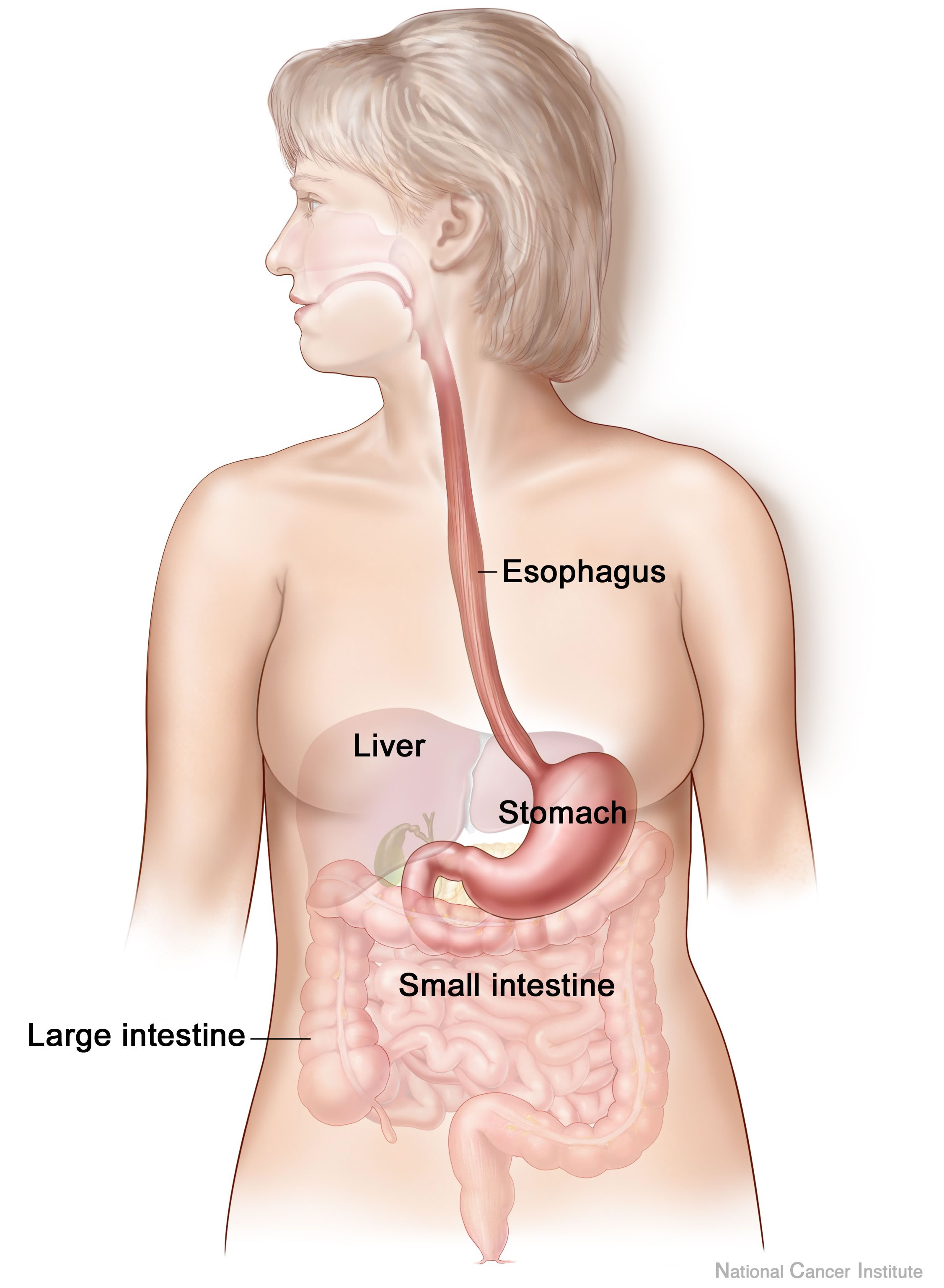
Digestive System
The human digestive system consists of the gastrointestinal tract plus the accessory organs of digestion (the tongue, salivary glands, pancreas, liver, and gallbladder). Digestion involves the breakdown of food into smaller and smaller components, until they can be absorbed and assimilated into the body. The process of digestion has three stages: the cephalic phase, the gastric phase, and the intestinal phase. The first stage, the cephalic phase of digestion, begins with secretions from gastric glands in response to the sight and smell of food. This stage includes the mechanical breakdown of food by chewing, and the chemical breakdown by digestive enzymes, that takes place in the mouth. Saliva contains the digestive enzymes amylase, and lingual lipase, secreted by the salivary and serous glands on the tongue. Chewing, in which the food is mixed with saliva, begins the mechanical process of digestion. This produces a bolus which is swallowed down the esophagus to enter the st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Digestion
Digestion is the breakdown of large insoluble food molecules into small water-soluble food molecules so that they can be absorbed into the watery blood plasma. In certain organisms, these smaller substances are absorbed through the small intestine into the blood stream. Digestion is a form of catabolism that is often divided into two processes based on how food is broken down: mechanical and chemical digestion. The term mechanical digestion refers to the physical breakdown of large pieces of food into smaller pieces which can subsequently be accessed by digestive enzymes. Mechanical digestion takes place in the mouth through mastication and in the small intestine through segmentation contractions. In chemical digestion, enzymes break down food into the small molecules the body can use. In the human digestive system, food enters the mouth and mechanical digestion of the food starts by the action of mastication (chewing), a form of mechanical digestion, and the wetting contact o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrochloric Acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid Acid strength is the tendency of an acid, symbolised by the chemical formula HA, to dissociate into a proton, H+, and an anion, A-. The dissociation of a strong acid in solution is effectively complete, except in its most concentrated solutions .... It is a component of the gastric acid in the digestive systems of most animal species, including humans. Hydrochloric acid is an important laboratory reagent and industrial chemical. History In the early tenth century, the Persian physician and alchemist Abu Bakr al-Razi ( 865–925, Latin: Rhazes) conducted experiments with sal ammoniac (ammonium chloride) and vitriol (hydrated sulfates of various metals), which he distilled together, thus producing the gas hydrogen chloride. In doing so, al-Razi may have stumbled upon a primitive method ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gastric Acid
Gastric acid, gastric juice, or stomach acid is a digestive fluid formed within the stomach lining. With a pH between 1 and 3, gastric acid plays a key role in digestion of proteins by activating digestive enzymes, which together break down the long chains of amino acids of proteins. Gastric acid is regulated in feedback systems to increase production when needed, such as after a meal. Other cells in the stomach produce bicarbonate, a base, to buffer the fluid, ensuring a regulated pH. These cells also produce mucus – a viscous barrier to prevent gastric acid from damaging the stomach. The pancreas further produces large amounts of bicarbonate and secretes bicarbonate through the pancreatic duct to the duodenum to neutralize gastric acid passing into the digestive tract. The active components of gastric acid are protons and chloride. Often simplistically described as hydrochloric acid, these species are produced by parietal cells in the gastric glands in the stomach. The sec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pepstatin
Pepstatin is a potent inhibitor of aspartyl proteases. It is a hexa-peptide containing the unusual amino acid statine (Sta, (3S,4S)-4-amino-3-hydroxy-6-methylheptanoic acid), having the sequence Isovaleryl-Val-Val-Sta-Ala-Sta (Iva-Val-Val-Sta-Ala-Sta). It was originally isolated from cultures of various species of Actinomyces due to its ability to inhibit pepsin at picomolar concentrations. Pepstatin A is well known to be an inhibitor of aspartic proteases such as pepsin, cathepsins D and E. Except for its role as a protease inhibitor, however, the pharmacological action of pepstatin A upon cells remain unclear. Pepstatin A suppresses receptor activator of NF-κB ligand (RANKL)–induced osteoclast differentiation. Pepstatin A suppresses the formation of multinuclear osteoclasts dose-dependently. This inhibition of the formation only affected osteoclast cells, i.e., not osteoblast-like cells. Furthermore, pepstatin A also suppresses differentiation from pre-osteoclast cells to mo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

_Schwann_(1810-1882)%2C_fondateur_de_la_théorie_cellulaire_(1839)%2C_Institut_de_Zoologie%2C_Liège.jpg)