|
Pentamethyltantalum
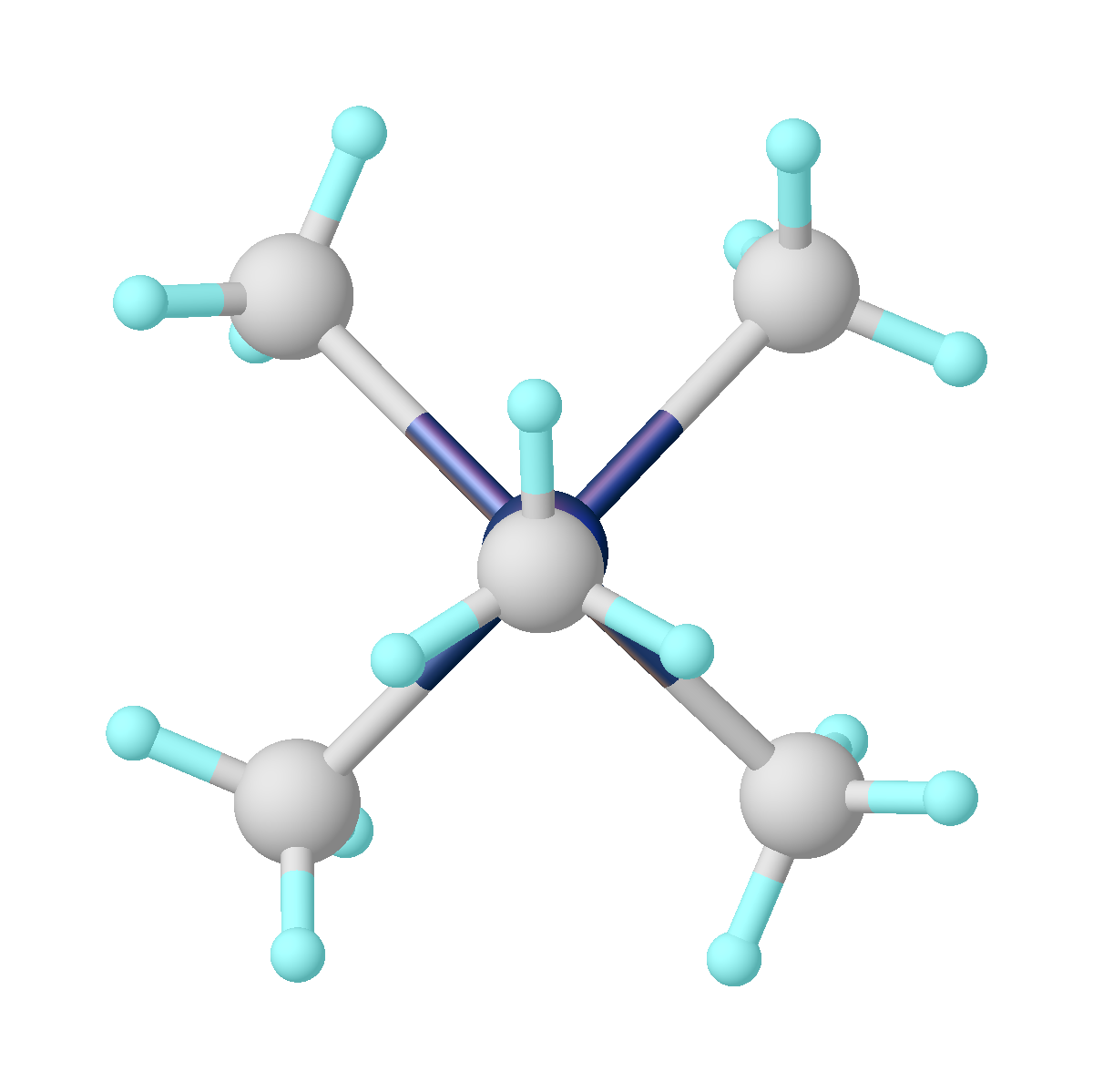
Pentamethyltantalum is a homoleptic organotantalum compound. It has a propensity to explode when it is melted. Its discovery was part of a sequence that lead to Richard R. Schrock's Nobel Prize discovery in olefin metathesis. Production Pentamethyltantalum can be made from the reaction of methyllithium with Ta(CH3)3Cl2. Ta(CH3)3Cl2 is in turn made from tantalum pentachloride and dimethylzinc. The preparation was inspired by the existence of pentaalkyl compounds of phosphorus and arsenic, and the discovery of hexamethyltungsten. The discoverer, Richard R. Schrock considered tantalum to be a metallic phosphorus, and tried the use of methyllithium. Properties The pentamethyltantalum adopts a square pyramid shape. Ignoring the C-H bonds, the molecule has ''C''4v symmetry. The four carbon atoms at the base of the pyramid are called basal, and the carbon atom at the top is called apical or apex. The distance from tantalum to the apical carbon atom is 2.11 Å, and to the basal car ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tantalum Compounds
Tantalum is a chemical element with the symbol Ta and atomic number 73. Previously known as ''tantalium'', it is named after Tantalus, a villain in Greek mythology. Tantalum is a very hard, ductile, lustrous, blue-gray transition metal that is highly corrosion-resistant. It is part of the refractory metals group, which are widely used as components of strong high-melting-point alloys. It is a group 5 element, along with vanadium and niobium, and it always occurs in geologic sources together with the chemically similar niobium, mainly in the mineral groups tantalite, columbite and coltan. The chemical inertness and very high melting point of tantalum make it valuable for laboratory and industrial equipment such as reaction vessels and vacuum furnaces. It is used in tantalum capacitors for electronic equipment such as computers. Tantalum is considered a technology-critical element by the European Commission. History Tantalum was discovered in Sweden in 1802 by Anders Ekebe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pentamethylarsenic
Pentamethylarsenic (or pentamethylarsorane)is an organometalllic compound containing five methyl groups bound to an arsenic atom with formula As(CH3)5. It is an example of a hypervalent compound. The molecular shape is trigonal bipyramid. History The first claim to make pentamethylarsenic was in 1862 in a reaction of tetramethylarsonium iodide with dimethylzinc by A. Cahours. For many years all the reproductions of this proved fruitless, so the production proved not to be genuine. It was actually discovered by Karl-Heinz Mitschke and Hubert Schmidbaur in 1973. Production Trimethylarsine is chlorinated to trimethylarsine dichloride, which then reacts with methyl lithium to yield pentamethylarsenic. :As(CH3)3 + Cl2 → As(CH3)3Cl2 :As(CH3)3Cl2 + 2LiCH3 → As(CH3)5 + 2LiCl Side products include As(CH3)4Cl and As(CH3)3=CH2. Pentamethylarsenic is not produced by biological organisms. Properties Pentamethylarsenic smells the same as pentamethylantimony, but is otherwise uniqu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pentamethylbismuth
Pentamethylbismuth (or pentamethylbismuthorane) is an organometalllic compound containing five methyl groups bound to a bismuth atom with formula Bi(CH3)5. It is an example of a hypervalent compound. The molecular shape is trigonal bipyramid. Production Pentamethylbismuth is produced in a two step process. First, trimethylbismuth is reacted with sulfuryl chloride to yield dichloro trimethylbismuth, which is then reacted with two equivalents of methyllithium dissolved in ether. The blue solution is cooled to −110 °C to precipitate the solid product. :Bi(CH3)3 + SO2Cl2 → Bi(CH3)3Cl2 + SO2 :Bi(CH3)3Cl2 + 2LiCH3 → Bi(CH3)5 + 2LiCl Properties At -110 °C, Bi(CH3)5 is a blue-violet solid. The methyl groups are arranged in a trigonal bipyramid, and the bond-lengths of methyl with bismuth are all the same. However, the molecule is not rigid, as can be determined from the nuclear magnetic resonance spectrum that shows all methyl groups are equivalent. It is stable as a solid, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pentamethylantimony
Pentamethylantimony or pentamethylstiborane is an organometalllic compound containing five methyl groups bound to an antimony atom with formula Sb(CH3)5. It is an example of a hypervalent compound. The molecular shape is trigonal bipyramid. Some other antimony(V) organometallic compounds include pentapropynylantimony (Sb(CCCH3)5) and pentaphenyl antimony (Sb(C6H5)5). Other known pentamethyl-pnictides include pentamethylbismuth and pentamethylarsenic. Production Pentamethylantimony can be made by reacting Sb(CH3)3Br2 with two equivalents of methyl lithium. Another production route is to convert trimethylstibine to the trimethyl antimony dichloride, and then replace the chlorine with methyl groups with methyl lithium. :Sb(CH3)3 + Cl2 → Sb(CH3)3Cl2 :Sb(CH3)3Cl2 + 2LiCH3 → Sb(CH3)5 + 2LiCl Properties Pentamethylantimony is colourless. At -143 °C it crystallizes in the orthorhombic system with space group ''Ccmm''. Unit cell dimensions are a=6.630 Å b=11.004 � ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homoleptic
In inorganic chemistry, a homoleptic chemical compound is a metal compound with all ligands identical. The term uses the " homo-" prefix to indicate that something is the same for all. Any metal species which has more than one type of ligand is heteroleptic. Some compounds with names that suggest that they are homoleptic are in fact heteroleptic, because they have ligands in them which are not featured in the name. For instance dialkyl magnesium complexes, which are found in the equilibrium which exists in a solution of a Grignard reagent in an ether, have two ether ligands attached to each magnesium centre. Another example is a solution of trimethyl aluminium in an ether solvent (such as THF); similar chemistry should be expected for a triaryl or trialkyl borane. It is possible for some ligands such as DMSO to bind with two or more different coordination modes. It would still be reasonable to consider a complex which has only one type of ligand but with different coordination m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organotantalum Chemistry
Organotantalum chemistry is the chemistry of chemical compounds containing a carbon-to-tantalum chemical bond. A wide variety of compound have been reported, initially with cyclopentadienyl and CO ligands. Oxidation states vary from Ta(V) to Ta(-I). Classes of organotantalum compounds Alkyl and aryl complexes Pentamethyltantalum was reported by Richard Schrock in 1974. Salts of a(CH3)6sup>− are prepared by alkylation of TaF5 using methyl lithium: :TaF5 + 6 LiCH3 → Li a(CH3)6+ 5 LiF Alkylidene complexes Tantalum alkylidene complexes arise by treating trialkyltantalum dichloride with alkyl lithium reagents. This reaction initially forms a thermally unstable tetraalkyl-monochloro-tantalum complex, which undergoes α-hydrogen elimination, followed by alkylation of the remaining chloride. Tantalum alkylidene complexes are nucleophilic. They effect a number of reactions including: olefinations, olefin metathesis, hydroaminoalkylation of olefins, and conju ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Richard R
Richard is a male given name. It originates, via Old French, from Old Frankish and is a compound of the words descending from Proto-Germanic ''*rīk-'' 'ruler, leader, king' and ''*hardu-'' 'strong, brave, hardy', and it therefore means 'strong in rule'. Nicknames include "Richie", "Dick", "Dickon", " Dickie", "Rich", "Rick", "Rico", "Ricky", and more. Richard is a common English, German and French male name. It's also used in many more languages, particularly Germanic, such as Norwegian, Danish, Swedish, Icelandic, and Dutch, as well as other languages including Irish, Scottish, Welsh and Finnish. Richard is cognate with variants of the name in other European languages, such as the Swedish "Rickard", the Catalan "Ricard" and the Italian "Riccardo", among others (see comprehensive variant list below). People named Richard Multiple people with the same name * Richard Andersen (other) * Richard Anderson (other) * Richard Cartwright (other) * Ri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Olefin Metathesis
Olefin metathesis is an organic reaction that entails the redistribution of fragments of alkenes (olefins) by the scission and regeneration of carbon-carbon double bonds. Because of the relative simplicity of olefin metathesis, it often creates fewer undesired by-products and hazardous wastes than alternative organic reactions. For their elucidation of the reaction mechanism and their discovery of a variety of highly active catalysts, Yves Chauvin, Robert H. Grubbs, and Richard R. Schrock were collectively awarded the 2005 Nobel Prize in Chemistry. Catalysts The reaction requires metal catalysts. Most commercially important processes employ heterogeneous catalysts. The heterogeneous catalysts are often prepared by in-situ activation of a metal halides (MClx) using organoaluminium or organotin compounds, e.g. combining MClx–EtAlCl2. A typical catalyst support is alumina. Commercial catalysts are often based on molybdenum and ruthenium. Well-defined organometallic co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tantalum Pentachloride
Tantalum(V) chloride, also known as tantalum pentachloride, is an inorganic compound with the formula TaCl5. It takes the form of a white powder and is commonly used as a starting material in tantalum chemistry. It readily hydrolyzes to form tantalum(V) oxychloride (TaOCl3) and eventually tantalum pentoxide (Ta2O5); this requires that it be synthesised and manipulated under anhydrous conditions, using air-free techniques. Structure TaCl5 crystallizes in the monoclinic space group ''C''2/''m''. The ten chlorine atoms define a pair of octahedra that share a common edge. The tantalum atoms occupy the centres of the octahedra and are joined by two chlorine bridging ligands. The dimeric structure is retained in non-complexing solvents and to a large extent in the molten state. In the vapour state, however, TaCl5 is monomeric. This monomer adopts a trigonal bipyramidal structure, like that of PCl5. Physical Properties The solubility of tantalum pentachloride increases slightly for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethylzinc
Dimethylzinc, also known as Zinc methyl, DMZ, or DMZn is a colorless volatile liquid Zn(CH3)2, formed by the action of methyl iodide on zinc at elevated temperature or on zinc sodium alloy. :2Zn + 2CH3I → Zn(CH3)2 + ZnI2 The sodium assists the reaction of the zinc with the methyl iodide. Zinc iodide is formed as a byproduct. It has a disagreeable odor, and is pyrophoric. It has been of great importance in the synthesis of organic compounds. It is soluble in alkanes and often sold as a solution in hexanes. It belongs to the large series of similar compounds such as diethylzinc. History This substance was first prepared by Edward Frankland during his work with Robert Bunsen in 1849 at the University of Marburg. After heating a mixture of zinc and methyl iodide in an airtight vessel, a flame burst out when the seal was broken. In the laboratory, this synthesis method remains unchanged today, except that copper or copper compounds are used to activate the zinc. Uses Dimethyl zi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexamethyltungsten
Hexamethyltungsten is the chemical compound Tungsten, W(Methyl, CH3)6 also written WMe6. Classified as a transition metal alkyl complexes, transition metal alkyl complex, hexamethyltungsten is an air-sensitive, red, crystalline solid at room temperature; however, it is extremely volatile and sublimes at −30 °C. Owing to its six methyl groups it is extremely soluble in petroleum, aromatic hydrocarbons, ethers, carbon disulfide, and carbon tetrachloride. Synthesis Hexamethyltungsten was first reported in 1973 by Geoffrey Wilkinson, Wilkinson and Shortland, who described its preparation by the reaction of methyllithium with tungsten hexachloride in diethyl ether. The synthesis was motivated in part by previous work which indicated that tetrahedral methyl transition metal compounds are thermally unstable, in the hopes that an octahedral molecular geometry, octahedral methyl compound would prove to be more robust. In 1976, Wilkinson and Galyer disclosed an improved synthesis usi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |