|
Palmitoyl-protein Thioesterase
Palmitoyl protein hydrolase/thioesterases is an enzyme (EC 3.1.2.22) that removes thioester-linked fatty acyl groups such as palmitate from modified cysteine residues in proteins or peptides during lysosomal degradation. It catalyzes the reaction :palmitoyl rotein+ H2O \rightleftharpoons palmitate + protein This enzyme belongs to the family of hydrolases, specifically those acting on thioester bonds. The systematic name is palmitoyl roteinhydrolase. Other names in common use include palmitoyl-protein thioesterase, and palmitoyl-(protein) hydrolase. This enzyme participates in fatty acid elongation in mitochondria. Neuronal ceroid lipofuscinoses (NCL) represent a group of encephalopathies that occur in 1 in 12,500 children. Mutations in the palmitoyl protein thioesterase gene causing infantile neuronal ceroid lipofuscinosis. The most common mutation results in intracellular accumulation of the polypeptide and undetectable enzyme activity in the brain. Direct sequencing of c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lipid Bilayer
The lipid bilayer (or phospholipid bilayer) is a thin polar membrane made of two layers of lipid molecules. These membranes are flat sheets that form a continuous barrier around all cells. The cell membranes of almost all organisms and many viruses are made of a lipid bilayer, as are the nuclear membrane surrounding the cell nucleus, and membranes of the membrane-bound organelles in the cell. The lipid bilayer is the barrier that keeps ions, proteins and other molecules where they are needed and prevents them from diffusing into areas where they should not be. Lipid bilayers are ideally suited to this role, even though they are only a few nanometers in width, because they are impermeable to most water-soluble (hydrophilic) molecules. Bilayers are particularly impermeable to ions, which allows cells to regulate salt concentrations and pH by transporting ions across their membranes using proteins called ion pumps. Biological bilayers are usually composed of amphiphilic phosphol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PPT1
Palmitoyl-protein thioesterase 1 (PPT-1), also known as palmitoyl-protein hydrolase 1, is an enzyme that in humans is encoded by the PPT1 gene. Function PPT-1 a member of the palmitoyl protein thioesterase family. PPT-1 is a small glycoprotein involved in the catabolism of lipid-modified proteins during lysosomal degradation. This enzyme removes thioester-linked fatty acyl groups such as palmitate from cysteine residues. Clinical significance Defects in this gene are a cause of neuronal ceroid lipofuscinosis type 1 (CLN1). Genetic basis and phenotypic correlations of the neuronal ceroid lipofusinoses. eview Warrier V; Vieira M; Mole SE. Biochimica et Biophysica Acta. 1832(11):1827-30, 2013 References Further reading *Acyl-protein thioesterase Acyl-protein thioesterases are enzymes that cleave off lipid modifications on proteins, located on the sulfur atom of cysteine residues linked via a thioester bond. Acyl-protein thioesterases are part of the α/β hydrolase ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acyl-protein Thioesterase
Acyl-protein thioesterases are enzymes that cleave off lipid modifications on proteins, located on the sulfur atom of cysteine residues linked via a thioester bond. Acyl-protein thioesterases are part of the α/β hydrolase superfamily of proteins and have a conserved catalytic triad. For that reason, acyl-protein thioesterases are also able to hydrolyze oxygen-linked ester bonds. Function Acyl-protein thioesterases are involved in the depalmitoylation of proteins, meaning they cleave off palmitoyl modifications on proteins' cysteine residues. Cellular targets include trimeric G-alpha proteins, ion channels and GAP-43. Moreover, human acyl-protein thioesterases 1 and 2 have been identified as major components in controlling the palmitoylation cycle of the oncogene Ras. Depalmitoylation of Ras by acyl-protein thioesterases potentially reduces Ras' affinity to endomembranes, allowing it to be palmitoylated again at the Golgi apparatus and to be directed to the plasma membr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Palmitoyl
Palmitoylation is the covalent attachment of fatty acids, such as palmitic acid, to cysteine (''S''-palmitoylation) and less frequently to serine and threonine (''O''-palmitoylation) residues of proteins, which are typically membrane proteins. The precise function of palmitoylation depends on the particular protein being considered. Palmitoylation enhances the hydrophobicity of proteins and contributes to their membrane association. Palmitoylation also appears to play a significant role in subcellular trafficking of proteins between membrane compartments, as well as in modulating protein–protein interactions. In contrast to prenylation and myristoylation, palmitoylation is usually reversible (because the bond between palmitic acid and protein is often a thioester bond). The reverse reaction in mammalian cells is catalyzed by acyl-protein thioesterases (APTs) in the cytosol and palmitoyl protein thioesterases in lysosomes. Because palmitoylation is a dynamic, post-translat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Data Bank
The Protein Data Bank (PDB) is a database for the three-dimensional structural data of large biological molecules, such as proteins and nucleic acids. The data, typically obtained by X-ray crystallography, NMR spectroscopy, or, increasingly, cryo-electron microscopy, and submitted by biologists and biochemists from around the world, are freely accessible on the Internet via the websites of its member organisations (PDBe, PDBj, RCSB, and BMRB). The PDB is overseen by an organization called the Worldwide Protein Data Bank, wwPDB. The PDB is a key in areas of structural biology, such as structural genomics. Most major scientific journals and some funding agencies now require scientists to submit their structure data to the PDB. Many other databases use protein structures deposited in the PDB. For example, SCOP and CATH classify protein structures, while PDBsum provides a graphic overview of PDB entries using information from other sources, such as Gene ontology. History Two force ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tertiary Structure
Protein tertiary structure is the three dimensional shape of a protein. The tertiary structure will have a single polypeptide chain "backbone" with one or more protein secondary structures, the protein domains. Amino acid side chains may interact and bond in a number of ways. The interactions and bonds of side chains within a particular protein determine its tertiary structure. The protein tertiary structure is defined by its atomic coordinates. These coordinates may refer either to a protein domain or to the entire tertiary structure.Branden C. and Tooze J. "Introduction to Protein Structure" Garland Publishing, New York. 1990 and 1991. A number of tertiary structures may fold into a quaternary structure.Kyte, J. "Structure in Protein Chemistry." Garland Publishing, New York. 1995. History The science of the tertiary structure of proteins has progressed from one of hypothesis to one of detailed definition. Although Emil Fischer had suggested proteins were made of polypept ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PPT2
Lysosomal thioesterase PPT2 (PPT-2), also known as S-thioesterase G14, is an enzyme that in humans is encoded by the ''PPT2'' gene. Function This gene encodes a member of the palmitoyl protein thioesterase family. The encoded glycosylated lysosomal protein has palmitoyl-CoA hydrolase activity in vitro, but does not hydrolyze palmitate from cysteine Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula . The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile. When present as a deprotonated catalytic residue, sometime ... residues in proteins. References Further reading * * * * * * * * * * * * * * * * * EC 3.1.2 {{gene-6-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neuronal Ceroid Lipofuscinosis
Neuronal ceroid lipofuscinosis is the general name for a family of at least eight genetically separate neurodegenerative lysosomal storage diseases that result from excessive accumulation of lipopigments (lipofuscin) in the body's tissues. These lipopigments are made up of fats and proteins. Their name comes from the word stem "lipo-", which is a variation on lipid, and from the term "pigment", used because the substances take on a greenish-yellow color when viewed under an ultraviolet light microscope. These lipofuscin materials build up in neuronal cells and many organs, including the liver, spleen, myocardium, and kidneys. Signs and symptoms The classic characterization of the group of neurodegenerative, lysosomal storage disorders called the neuronal ceroid lipofuscinoses (NCLs) is through the progressive, permanent loss of motor and psychological ability with a severe intracellular accumulation of lipofuscins, with the United States and Northern European populations having sli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
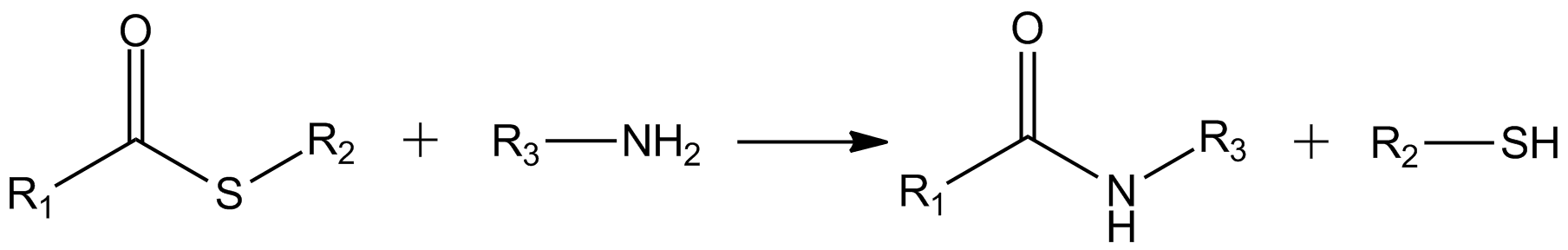
Thioester
In organic chemistry, thioesters are organosulfur compounds with the functional group . They are analogous to carboxylate esters () with the sulfur in the thioester playing the role of the linking oxygen in the carboxylate ester, as implied by the ''thio-'' prefix. They are the product of esterification between a carboxylic acid () and a thiol (). In biochemistry, the best-known thioesters are derivatives of coenzyme A, e.g., acetyl-CoA.Matthys J. Janssen "Carboxylic Acids and Esters" in PATAI's Chemistry of Functional Groups: Carboxylic Acids and Esters, Saul Patai, Ed. John Wiley, 1969, New York: pp. 705–764. Synthesis The most typical route to thioester involves the reaction of an acid chloride with an alkali metal salt of a thiol: :RSNa + R'COCl -> R'COSR + NaCl Another common route entails the displacement of halides by the alkali metal salt of a thiocarboxylic acid. For example, thioacetate esters are commonly prepared by alkylation of potassium thioacetate: :CH3COSK + ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fatty Acid Elongation In Mitochondria
Fatty is a derogatory term for someone who is obese. It may refer also to: People * Mai Fatty, Gambian politician * Roscoe Arbuckle (1887–1933), American actor and comedian * Fatty Briody (1858–1903), American Major League Baseball player * Fatty D (April Fores), American pornographic actress * Bob Fothergill (1897–1938), American Major League Baseball outfielder * William Foulke (footballer) (1874–1916), English cricketer and footballer * Fatty George (1927–1982), Austrian jazz musician * Richard Lamb (1907–1974), Australian racing cyclist * Fatty Lawrence (1903–1976), college gridiron football player * W. T. McLain (1885–1938), college gridiron football player, lawyer, and politician * Charles H. Smith (American football), University of Michigan football player in 1893–1894 * Roland Taylor (1946–2017), retired American Basketball Association and National Basketball Association player * Paul Vautin (born 1959), Australian former rugby league footballer and co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of Enzymes
This article lists enzymes by their classification in the International Union of Biochemistry and Molecular Biology's Enzyme Commission (EC) numbering system. * List of EC numbers (EC 5) * List of EC numbers (EC 6) :Oxidoreductases (EC 1) (Oxidoreductase) *Dehydrogenase * Luciferase *DMSO reductase :EC 1.1 (act on the CH-OH group of donors) * :EC 1.1.1 (with NAD+ or NADP+ as acceptor) ** Alcohol dehydrogenase (NAD) ** Alcohol dehydrogenase (NADP) **Homoserine dehydrogenase ** Aminopropanol oxidoreductase **Diacetyl reductase **Glycerol dehydrogenase **Propanediol-phosphate dehydrogenase ** glycerol-3-phosphate dehydrogenase (NAD+) ** D-xylulose reductase **L-xylulose reductase **Lactate dehydrogenase **Malate dehydrogenase **Isocitrate dehydrogenase ** HMG-CoA reductase * :EC 1.1.2 (with a cytochrome as acceptor) * :EC 1.1.3 (with oxygen as acceptor) **Glucose oxidase **L-gulonolactone oxidase **Thiamine oxidase **Xanthine oxidase * :EC 1.1.4 (with a disul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |