|
Oxyanions
An oxyanion, or oxoanion, is an ion with the generic formula (where A represents a chemical element and O represents an oxygen atom). Oxyanions are formed by a large majority of the chemical elements. The formulae of simple oxyanions are determined by the octet rule. The corresponding oxyacid of an oxyanion is the compound . The structures of condensed oxyanions can be rationalized in terms of AO''n'' polyhedral units with sharing of corners or edges between polyhedra. The oxyanions (specifically, phosphate and polyphosphate esters) adenosine monophosphate (adenosine monophosphate, AMP), adenosine diphosphate (adenosine diphosphate, ADP) and adenosine triphosphate (ATP) are important in biology. Monomeric oxyanions The formula of monomeric oxyanions, , is dictated by the oxidation state of the element A and its position in the periodic table. Elements of the first row are limited to a maximum coordination number of 4. However, none of the first row elements has a monomeric oxyanio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
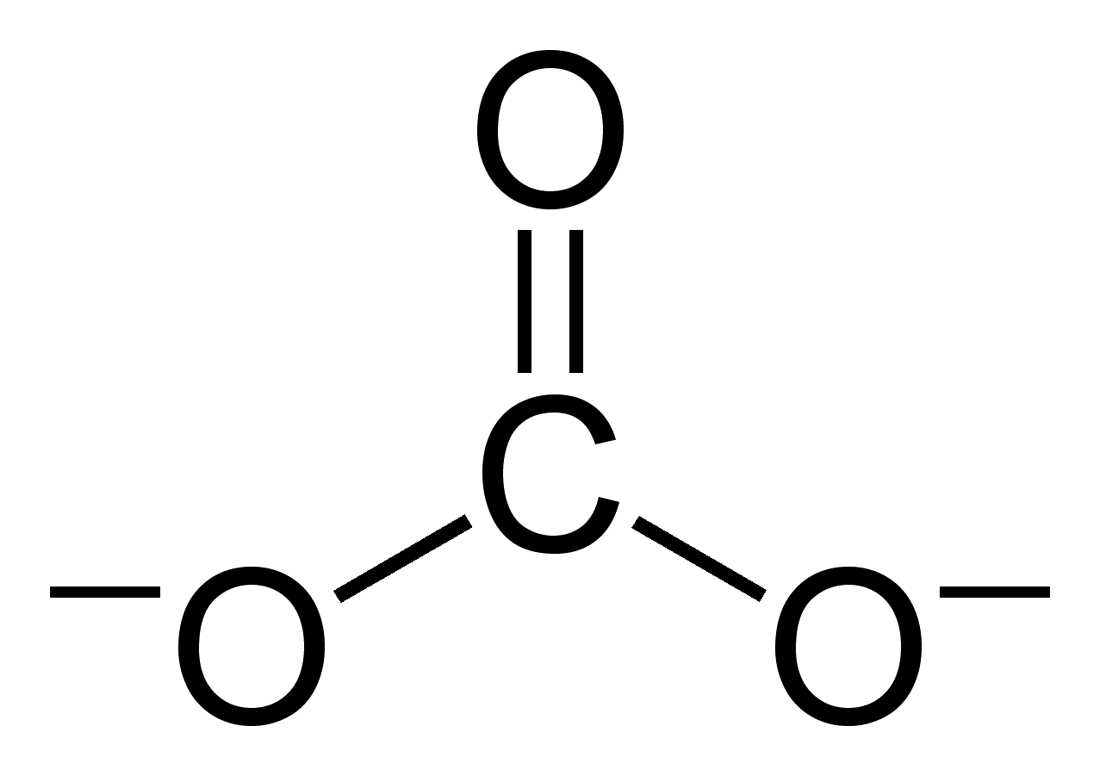
Carbonate
A carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word ''carbonate'' may also refer to a carbonate ester, an organic compound containing the carbonate group C(=O)(O–)2. The term is also used as a verb, to describe carbonation: the process of raising the concentrations of carbonate and bicarbonate ions in water to produce carbonated water and other carbonated beverageseither by the addition of carbon dioxide gas under pressure or by dissolving carbonate or bicarbonate salts into the water. In geology and mineralogy, the term "carbonate" can refer both to carbonate minerals and carbonate rock (which is made of chiefly carbonate minerals), and both are dominated by the carbonate ion, . Carbonate minerals are extremely varied and ubiquitous in chemically precipitated sedimentary rock. The most common are calcite or calcium carbonate, CaCO3, the chief constituent of limestone (as well a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Borate
A borate is any of several boron oxyanions, negative ions consisting of boron and oxygen, such as orthoborate , metaborate , or tetraborate ; or any salt with such anions, such as sodium metaborate, and disodium tetraborate . The name also refers to certain functional groups in molecules consisting of boron and oxygen, and esters with such groups, such as triethyl orthoborate . Natural occurrence Borate ions occur, alone or with other anions, in many borate and borosilicate minerals such as borax, boracite, ulexite (boronatrocalcite) and colemanite. Borates also occur in seawater, where they make an important contribution to the absorption of low frequency sound in seawater. Borates also occur in plants, including almost all fruits. Anions The main borate anions are: * tetrahydroxyborate , found in sodium tetrahydroxyborate . * orthoborate , found in trisodium orthoborate * perborate , as in sodium perborate * metaborate or , found in sodium metaborate * diborate , f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molybdate
In chemistry a molybdate is a compound containing an oxoanion with molybdenum in its highest oxidation state of 6. Molybdenum can form a very large range of such oxoanions which can be discrete structures or polymeric extended structures, although the latter are only found in the solid state. The larger oxoanions are members of group of compounds termed polyoxometalates, and because they contain only one type of metal atom are often called isopolymetalates. The discrete molybdenum oxoanions range in size from the simplest , found in potassium molybdate up to extremely large structures found in isopoly-molybdenum blues that contain for example 154 Mo atoms. The behaviour of molybdenum is different from the other elements in group 6. Chromium only forms the chromates, , , and ions which are all based on tetrahedral chromium. Tungsten is similar to molybdenum and forms many tungstates containing 6 coordinate tungsten. Examples of molybdate anions Examples of molybdate oxoanion ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonate
A carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word ''carbonate'' may also refer to a carbonate ester, an organic compound containing the carbonate group C(=O)(O–)2. The term is also used as a verb, to describe carbonation: the process of raising the concentrations of carbonate and bicarbonate ions in water to produce carbonated water and other carbonated beverageseither by the addition of carbon dioxide gas under pressure or by dissolving carbonate or bicarbonate salts into the water. In geology and mineralogy, the term "carbonate" can refer both to carbonate minerals and carbonate rock (which is made of chiefly carbonate minerals), and both are dominated by the carbonate ion, . Carbonate minerals are extremely varied and ubiquitous in chemically precipitated sedimentary rock. The most common are calcite or calcium carbonate, CaCO3, the chief constituent of limestone (as well a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrate
Nitrate is a polyatomic ion A polyatomic ion, also known as a molecular ion, is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that has a net charge that is not zero. The term molecule may or may no ... with the chemical formula . salt (chemistry), Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are solubility, soluble in water. An example of an insoluble nitrate is bismuth oxynitrate. Structure The ion is the conjugate acid, conjugate base of nitric acid, consisting of one central nitrogen atom surrounded by three identically bonded oxygen atoms in a trigonal planar arrangement. The nitrate ion carries a formal charge of −1. This charge results from a combination formal charge in which each of the three oxygens carries a − charge, whereas the nitrogen carries a +1 charge, all these adding up to formal c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfate
The sulfate or sulphate ion is a polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many are prepared from that acid. Spelling "Sulfate" is the spelling recommended by IUPAC, but "sulphate" was traditionally used in British English. Structure The sulfate anion consists of a central sulfur atom surrounded by four equivalent oxygen atoms in a tetrahedral arrangement. The symmetry is the same as that of methane. The sulfur atom is in the +6 oxidation state while the four oxygen atoms are each in the −2 state. The sulfate ion carries an overall charge of −2 and it is the conjugate base of the bisulfate (or hydrogensulfate) ion, , which is in turn the conjugate base of , sulfuric acid. Organic sulfate esters, such as dimethyl sulfate, are covalent compounds and esters of sulfuric acid. The tetrahedral molecular geometry of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orthoperiodate
Periodate is an anion composed of iodine and oxygen. It is one of a number of oxyanions of iodine and is the highest in the series, with iodine existing in oxidation state +7. Unlike other perhalogenates, such as perchlorate, it can exist in two forms: metaperiodate and orthoperiodate . In this regard it is comparable to the tellurate ion from the adjacent group. It can combine with a number of counter ions to form periodates, which may also be regarded as the salts of periodic acid. Periodates were discovered by Heinrich Gustav Magnus and C. F. Ammermüller; who first synthesised periodic acid in 1833. Synthesis Classically, periodate was most commonly produced in the form of sodium hydrogen periodate (). This is commercially available, but can also be produced by the oxidation of iodates with chlorine and sodium hydroxide. Or, similarly, from iodides by oxidation with bromine and sodium hydroxide: :\overset + Cl2 + 4 NaOH -> Na3H2IO6 + 2NaCl + H2O :NaI + 4 Br2 + 10 NaOH -> ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorate
The chlorate anion has the formula ClO3-. In this case, the chlorine atom is in the +5 oxidation state. "Chlorate" can also refer to chemical compounds containing this anion; chlorates are the salts of chloric acid. "Chlorate", when followed by a Roman numeral in parentheses, e.g. chlorate (VII), refers to a particular oxyanion of chlorine. As predicted by valence shell electron pair repulsion theory, chlorate anions have trigonal pyramidal structures. Chlorates are powerful oxidizers and should be kept away from organics or easily oxidized materials. Mixtures of chlorate salts with virtually any combustible material (sugar, sawdust, charcoal, organic solvents, metals, etc.) will readily deflagrate. Chlorates were once widely used in pyrotechnics for this reason, though their use has fallen due to their instability. Most pyrotechnic applications that formerly used chlorates now use the more stable perchlorates instead. Structure and bonding The chlorate ion cannot be satisf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorite
The chlorite ion, or chlorine dioxide anion, is the halite with the chemical formula of . A chlorite (compound) is a compound that contains this group, with chlorine in the oxidation state of +3. Chlorites are also known as salts of chlorous acid. Compounds The free acid, chlorous acid HClO2, is the least stable oxoacid of chlorine and has only been observed as an aqueous solution at low concentrations. Since it cannot be concentrated, it is not a commercial product. The alkali metal and alkaline earth metal compounds are all colorless or pale yellow, with sodium chlorite (NaClO2) being the only commercially important chlorite. Heavy metal chlorites (Ag+, Hg+, Tl+, Pb2+, and also Cu2+ and ) are unstable and decompose explosively with heat or shock. Sodium chlorite is derived indirectly from sodium chlorate, NaClO3. First, the explosively unstable gas chlorine dioxide, ClO2 is produced by reducing sodium chlorate with a suitable reducing agent such as methanol, hydrogen perox ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |