|
Oxy-fuel
Oxy-fuel combustion is the process of burning a fuel using pure oxygen, or a mixture of oxygen and recirculated flue gas, instead of air. Since the nitrogen component of air is not heated, fuel consumption is reduced, and higher flame temperatures are possible. Historically, the primary use of oxy-fuel combustion has been in welding and cutting of metals, especially steel, since oxy-fuel allows for higher flame temperatures than can be achieved with an air-fuel flame. It has also received a lot of attention in recent decades as a potential carbon capture and storage technology. There is currently research being done in firing fossil fuel power plants with an oxygen-enriched gas mix instead of air. Almost all of the nitrogen is removed from input air, yielding a stream that is approximately 95% oxygen. Firing with pure oxygen would result in too high a flame temperature, so the mixture is diluted by mixing with recycled flue gas, or staged combustion. The recycled flue gas can also ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxyfuel CCS Fossil Fuel Power Plant Operation
Oxy-fuel combustion is the process of burning a fuel using pure oxygen, or a mixture of oxygen and recirculated flue gas, instead of air. Since the nitrogen component of air is not heated, fuel consumption is reduced, and higher flame temperatures are possible. Historically, the primary use of oxy-fuel combustion has been in welding and cutting of metals, especially steel, since oxy-fuel allows for higher flame temperatures than can be achieved with an air-fuel flame. It has also received a lot of attention in recent decades as a potential carbon capture and storage technology. There is currently research being done in firing fossil fuel power plants with an oxygen-enriched gas mix instead of air. Almost all of the nitrogen is removed from input air, yielding a stream that is approximately 95% oxygen. Firing with pure oxygen would result in too high a flame temperature, so the mixture is diluted by mixing with recycled flue gas, or staged combustion. The recycled flue gas can also ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Schwarze Pumpe Power Station
Schwarze Pumpe power station (german: Kraftwerk Schwarze Pumpe translated: ''Black Pump Power Station'') is a modern lignite (brown coal)–fired power station in the "Schwarze Pumpe" (Black Pump) district in Spremberg, Germany consisting of 2 × 800 megawatts (MW) units. The current plant came into service in 1997–1998 and was built by Siemens. The power station was sold by Vattenfall to the Czech energy group EPH and its financial partner PPF Investments on 30September 2016. The cooling towers are high and have an observation deck on top. The site has been a large-scale industrial site processing lignite since the DDR period, when it was first developed (since 1955). The DDR-era plant used the lignite to produce high temperature lignite coke for blast furnaces, coal gas to fire steam turbine electrical generation, motor fuels, and a variety of chemical feedstocks. Carbon capture and storage pilot plant Construction started on 26 May 2006, in the Schwarze Pu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
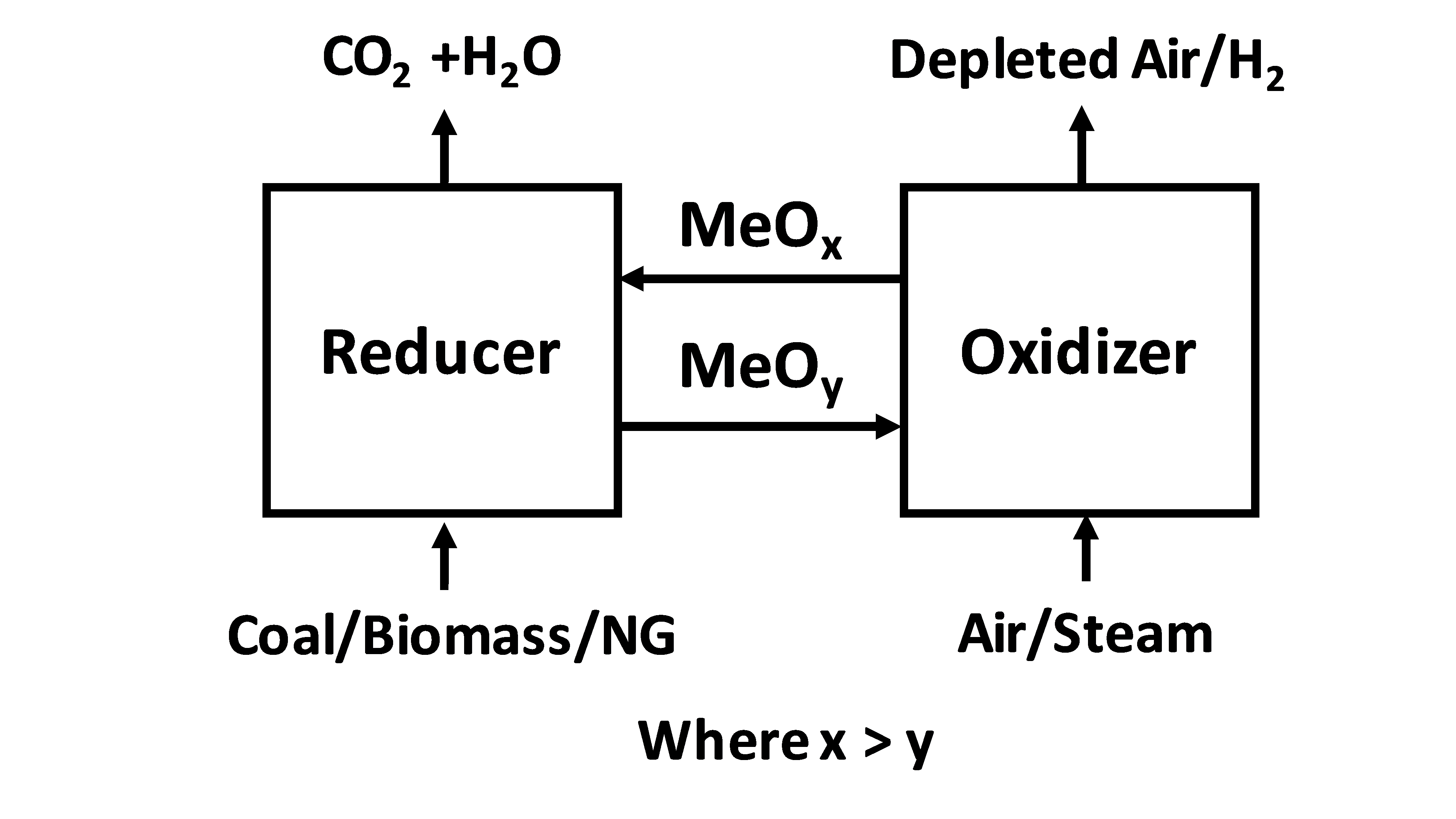
Chemical Looping Combustion
Chemical looping combustion (CLC) is a technological process typically employing a dual fluidized bed system. CLC operated with an interconnected moving bed with a fluidized bed system, has also been employed as a technology process. In CLC, a metal oxide is employed as a bed material providing the oxygen for combustion in the fuel reactor. The reduced metal is then transferred to the second bed ( air reactor) and re-oxidized before being reintroduced back to the fuel reactor completing the loop. Fig 1 shows a simplified diagram of the CLC process. Fig 2 shows an example of a dual fluidized bed circulating reactor system and a moving bed-fluidized bed circulating reactor system. Isolation of the fuel from air simplifies the number of chemical reactions in combustion. Employing oxygen without nitrogen and the trace gases found in air eliminates the primary source for the formation of nitrogen oxide (), produces a flue gas composed primarily of carbon dioxide and water vapor; other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Capture And Storage
Carbon capture and storage (CCS) or carbon capture and sequestration is the process of capturing carbon dioxide (CO2) before it enters the atmosphere, transporting it, and storing it (carbon sequestration) for centuries or millennia. Usually the CO2 is captured from large point sources, such as a chemical plant or biomass power plant, and then stored in an underground geological formation. The aim is to prevent the release of CO2 from heavy industry with the intent of mitigating the effects of climate change. CO2 has been injected into geological formations for several decades for enhanced oil recovery and after separation from natural gas, but this has been criticised for producing more emissions when the gas or oil is burned. Carbon capture and utilization (CCU) and CCS are sometimes discussed collectively as carbon capture, utilization, and sequestration (CCUS). This is because CCS is a relatively expensive process yielding a product which is often too cheap. Hence, carbon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
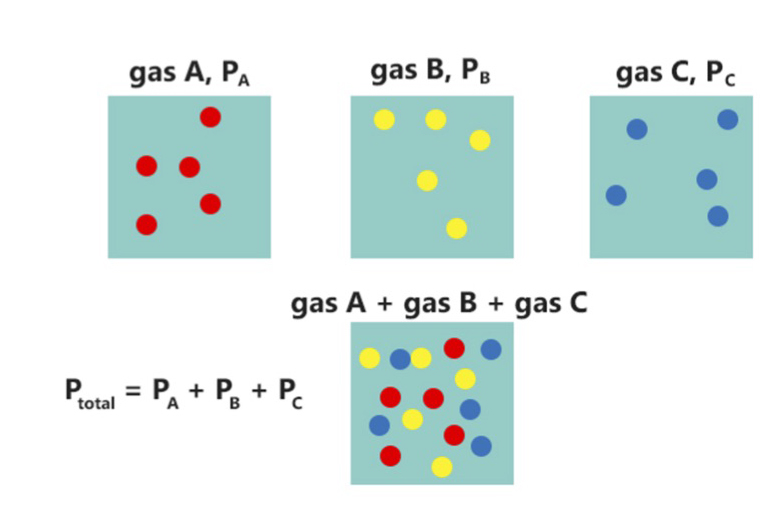
Partial Pressure
In a mixture of gases, each constituent gas has a partial pressure which is the notional pressure of that constituent gas as if it alone occupied the entire volume of the original mixture at the same temperature. The total pressure of an ideal gas mixture is the sum of the partial pressures of the gases in the mixture ( Dalton's Law). The partial pressure of a gas is a measure of thermodynamic activity of the gas's molecules. Gases dissolve, diffuse, and react according to their partial pressures but not according to their concentrations in gas mixtures or liquids. This general property of gases is also true in chemical reactions of gases in biology. For example, the necessary amount of oxygen for human respiration, and the amount that is toxic, is set by the partial pressure of oxygen alone. This is true across a very wide range of different concentrations of oxygen present in various inhaled breathing gases or dissolved in blood; consequently, mixture ratios, like that of breat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Equilibrium Reaction
In a chemical reaction, chemical equilibrium is the state in which both the reactants and products are present in concentrations which have no further tendency to change with time, so that there is no observable change in the properties of the system. This state results when the forward reaction proceeds at the same rate as the reverse reaction. The reaction rates of the forward and backward reactions are generally not zero, but they are equal. Thus, there are no net changes in the concentrations of the reactants and products. Such a state is known as dynamic equilibrium. Historical introduction The concept of chemical equilibrium was developed in 1803, after Berthollet found that some chemical reactions are reversible. For any reaction mixture to exist at equilibrium, the rates of the forward and backward (reverse) reactions must be equal. In the following chemical equation, arrows point both ways to indicate equilibrium. A and B are reactant chemical species, S and T are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcination
Calcination refers to thermal treatment of a solid chemical compound (e.g. mixed carbonate ores) whereby the compound is raised to high temperature without melting under restricted supply of ambient oxygen (i.e. gaseous O2 fraction of air), generally for the purpose of removing impurities or volatile substances and/or to incur thermal decomposition. The root of the word calcination refers to its most prominent use, which is to remove carbon from limestone (calcium carbonate) through combustion to yield calcium oxide (quicklime). This calcination reaction is CaCO3(s) → CaO(s) + CO2(g). Calcium oxide is a crucial ingredient in modern cement, and is also used as a chemical flux in smelting. Industrial calcination generally emits carbon dioxide (), making it a major contributor to climate change. A calciner is a steel cylinder that rotates inside a heated furnace and performs indirect high-temperature processing (550–1150 °C, or 1000–2100 °F) within a controlled ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Decomposition
Chemical decomposition, or chemical breakdown, is the process or effect of simplifying a single chemical entity (normal molecule, reaction intermediate, etc.) into two or more fragments. Chemical decomposition is usually regarded and defined as the exact opposite of chemical synthesis. In short, the chemical reaction in which two or more products are formed from a single reactant is called a decomposition reaction. The details of a decomposition process are not always well defined but some of the process is understood; much energy is needed to break bonds. Since all decomposition reactions break apart the bonds holding it together in order to produce into its simpler basic parts, the reactions would require some form of this energy in varying degrees. Because of this fundamental rule, it is known that most of these reactions are endothermic although exceptions do exist. The stability of a chemical compound is eventually limited when exposed to extreme environmental conditions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonate Mineral
Carbonate minerals are those minerals containing the carbonate ion, . Carbonate divisions Anhydrous carbonates *Calcite group: trigonal ** Calcite CaCO3 ** Gaspéite (Ni,Mg,Fe2+)CO3 ** Magnesite MgCO3 ** Otavite CdCO3 **Rhodochrosite MnCO3 **Siderite FeCO3 **Smithsonite ZnCO3 ** Spherocobaltite CoCO3 *Aragonite group: orthorhombic **Aragonite CaCO3 ** Cerussite PbCO3 ** Strontianite SrCO3 **Witherite BaCO3 **Rutherfordine UO2CO3 ** Natrite Na2CO3 Anhydrous carbonates with compound formulas *Dolomite group: trigonal ** Ankerite CaFe(CO3)2 ** Dolomite CaMg(CO3)2 ** Huntite Mg3Ca(CO3)4 ** Minrecordite CaZn(CO3)2 ** Barytocalcite BaCa(CO3)2 Carbonates with hydroxyl or halogen *Carbonate with hydroxide: monoclinic ** Azurite Cu3(CO3)2(OH)2 **Hydrocerussite Pb3(CO3)2(OH)2 ** Malachite Cu2CO3(OH)2 ** Rosasite (Cu,Zn)2CO3(OH)2 ** Phosgenite Pb2(CO3)Cl2 **Hydrozincite Zn5(CO3)2(OH)6 **Aurichalcite (Zn,Cu)5(CO3)2(OH)6 Hydrated carbonates *Hydromagnesite Mg5(CO3)4(OH)2.4H2O *Ikait ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NET Power Demonstration Facility
The NET Power Test Facility, located in La Porte, Tx, is an oxy-combustion, zero-emissions 50 MWth natural gas power plant owned and operated by NET Power. NET Power is owned by Constellation Energy Corporation, Occidental Petroleum Corporation Occidental Petroleum Corporation (often abbreviated Oxy in reference to its ticker symbol and logo) is an American company engaged in hydrocarbon exploration in the United States, and the Middle East as well as petrochemical manufacturing in the ... (Oxy) Low Carbon Ventures, Baker Hughes Company and 8 Rivers Capital, the company holding the patents for the technology. The plant is a first of its kind Allam-Fetvedt Cycle which achieved first-fire in May of 2018. The Allam-Fetvedt cycle delivers lower cost power while eliminating atmospheric emissions. The plant was featured in The Global CCS Institutes 2018 Status of CCS report. In recognition of the Allam-Fetvedt Cycle demonstration plant in La Porte, Texas, NET Power was awarded ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Hydroxide
Calcium hydroxide (traditionally called slaked lime) is an inorganic compound with the chemical formula Ca( OH)2. It is a colorless crystal or white powder and is produced when quicklime (calcium oxide) is mixed or slaked with water. It has many names including hydrated lime, caustic lime, builders' lime, slaked lime, cal, and pickling lime. Calcium hydroxide is used in many applications, including food preparation, where it has been identified as E number E526. Limewater, also called milk of lime, is the common name for a saturated solution of calcium hydroxide. Properties Calcium hydroxide is poorly soluble in water, with a retrograde solubility increasing from 0.66 g/L at 100 °C to 1.89 g/L at 0 °C. With a solubility product ''K''sp of 5.02 at 25 °C, its dissociation in water is large enough that its solutions are basic according to the following dissolution reaction: : Ca(OH)2 → Ca2+ + 2 OH− At ambient temperature, calcium hydroxide (portlandite) d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Oxide
Calcium oxide (CaO), commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline, crystalline solid at room temperature. The broadly used term "''lime''" connotes calcium-containing inorganic materials, in which carbonates, oxides and hydroxides of calcium, silicon, magnesium, aluminium, and iron predominate. By contrast, ''quicklime'' specifically applies to the single chemical compound calcium oxide. Calcium oxide that survives processing without reacting in building products such as cement is called free lime. Quicklime is relatively inexpensive. Both it and a chemical derivative ( calcium hydroxide, of which quicklime is the base anhydride) are important commodity chemicals. Preparation Calcium oxide is usually made by the thermal decomposition of materials, such as limestone or seashells, that contain calcium carbonate (CaCO3; mineral calcite) in a lime kiln. This is accomplished by heating the material to above ,Me ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |