|
Orotidine Monophosphate
Orotidine 5'-monophosphate (OMP), also known as orotidylic acid, is a pyrimidine nucleotide which is the last intermediate in the biosynthesis of uridine monophosphate. OMP is formed from orotate and phosphoribosyl pyrophosphate by the enzyme orotate phosphoribosyltransferase In humans, the enzyme UMP synthase The enzyme Uridine monophosphate synthase (, UMPS) (orotate phosphoribosyl transferase and orotidine-5'-decarboxylase) catalyses the formation of uridine monophosphate (UMP), an energy-carrying molecule in many important biosynthetic pathways. In h ... converts OMP into uridine 5'- monophosphate. If UMP synthase is defective, orotic aciduria can result. Nucleotides Pyrimidinediones {{biochem-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrimidine
Pyrimidine (; ) is an aromatic, heterocyclic, organic compound similar to pyridine (). One of the three diazines (six-membered heterocyclics with two nitrogen atoms in the ring), it has nitrogen atoms at positions 1 and 3 in the ring. The other diazines are pyrazine (nitrogen atoms at the 1 and 4 positions) and pyridazine (nitrogen atoms at the 1 and 2 positions). In nucleic acids, three types of nucleobases are pyrimidine derivatives: cytosine (C), thymine (T), and uracil (U). Occurrence and history The pyrimidine ring system has wide occurrence in nature as substituted and ring fused compounds and derivatives, including the nucleotides cytosine, thymine and uracil, thiamine (vitamin B1) and alloxan. It is also found in many synthetic compounds such as barbiturates and the HIV drug, zidovudine. Although pyrimidine derivatives such as alloxan were known in the early 19th century, a laboratory synthesis of a pyrimidine was not carried out until 1879, when Grimaux reported the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
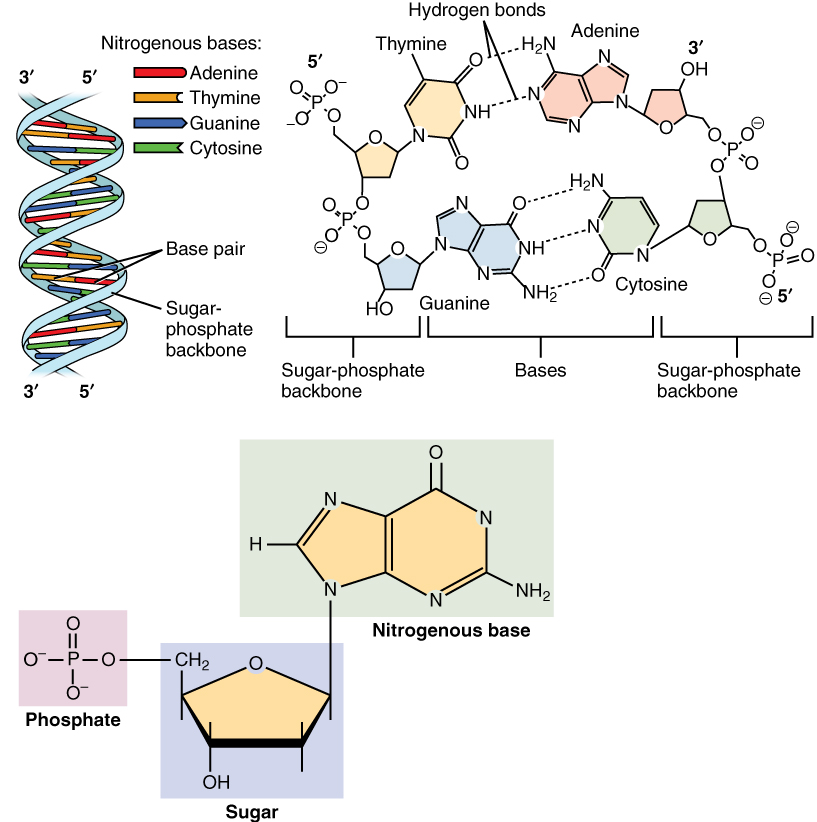
Nucleotide
Nucleotides are organic molecules consisting of a nucleoside and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both of which are essential biomolecules within all life-forms on Earth. Nucleotides are obtained in the diet and are also synthesized from common nutrients by the liver. Nucleotides are composed of three subunit molecules: a nucleobase, a five-carbon sugar (ribose or deoxyribose), and a phosphate group consisting of one to three phosphates. The four nucleobases in DNA are guanine, adenine, cytosine and thymine; in RNA, uracil is used in place of thymine. Nucleotides also play a central role in metabolism at a fundamental, cellular level. They provide chemical energy—in the form of the nucleoside triphosphates, adenosine triphosphate (ATP), guanosine triphosphate (GTP), cytidine triphosphate (CTP) and uridine triphosphate (UTP)—throughout the cell for the many cellular func ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uridine Monophosphate
Uridine monophosphate (UMP), also known as 5′-uridylic acid (conjugate base uridylate), is a nucleotide that is used as a monomer in RNA. It is an ester of phosphoric acid with the nucleoside uridine. UMP consists of the phosphate group, the pentose sugar ribose, and the nucleobase uracil; hence, it is a ribonucleotide monophosphate. As a substituent or radical its name takes the form of the prefix uridylyl-. The deoxy form is abbreviated dUMP. Covalent attachment of UMP (e.g. to a protein such as adenylyltransferase) is called uridylylation (or sometimes uridylation). Biosynthesis Uridine monophosphate is formed from Orotidine 5'-monophosphate (orotidylic acid) in a decarboxylation reaction catalyzed by the enzyme orotidylate decarboxylase. Uncatalyzed, the decarboxylation reaction is extremely slow (estimated to occur on average one time per 78 million years). Adequately catalyzed, the reaction takes place once per second, an increase of 1017-fold. In humans, the orotidyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orotic Acid
Orotic acid is a pyrimidinedione and a carboxylic acid. Historically, it was believed to be part of the vitamin B complex and was called vitamin B13, but it is now known that it is not a vitamin. The compound is synthesized in the body via a mitochondrial enzyme, dihydroorotate dehydrogenase or a cytoplasmic enzyme of Pyrimidine metabolism, pyrimidine synthesis pathway. It is sometimes used as a mineral carrier in some dietary supplements (to increase their bioavailability), most commonly for lithium orotate. Synthesis Dihydroorotate is synthesized to orotic acid by the enzyme dihydroorotate dehydrogenase, where it later combines with phosphoribosyl pyrophosphate (PRPP) to form Orotidine 5'-monophosphate, orotidine-5'-monophosphate (OMP). A distinguishing characteristic of pyrimidine synthesis is that the pyrimidine ring is fully synthesized before being attached to the ribose sugar, whereas purine synthesis happens by building the base directly on the sugar. Chemistry Orotic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphoribosyl Pyrophosphate
Phosphoribosyl pyrophosphate (PRPP) is a pentose phosphate. It is a biochemical intermediate in the formation of purine nucleotides via inosine-5-monophosphate, as well as in pyrimidine nucleotide formation. Hence it is a building block for DNA and RNA. The vitamins thiamine and cobalamin, and the amino acid tryptophan also contain fragments derived from PRPP. It is formed from ribose 5-phosphate (R5P) by the enzyme ribose-phosphate diphosphokinase: : It plays a role in transferring phospho-ribose groups in several reactions, some of which are salvage pathways: In '' de novo'' generation of purines, the enzyme amidophosphoribosyltransferase acts upon PRPP to create phosphoribosylamine. The histidine biosynthesis pathway involves the reaction between PRPP and ATP, which activates the latter to ring cleavage. Carbon atoms from ribose in PRPP form the linear chain and part of the imidazole ring in histidine. The same is true for the biosynthesis of tryptophan, with the first s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orotate Phosphoribosyltransferase
Orotate phosphoribosyltransferase (OPRTase) or orotic acid phosphoribosyltransferase is an enzyme involved in pyrimidine biosynthesis. It catalyzes the formation of orotidine 5'-monophosphate (OMP) from orotate and phosphoribosyl pyrophosphate. In yeast and bacteria, orotate phosphoribosyltransferase is an independent enzyme with a unique gene coding for the protein, whereas in mammals and other multicellular organisms, the catalytic function is carried out by a domain of the bifunctional enzyme UMP synthase (UMPS). Biological background As OPRTase is part of a bifunctional complex UMP synthase in humans, the function and stability of this enzyme is not necessarily directly associated with disorders in the human body. It is however reasonable to believe that a dysfunction in one of the enzymes will cause a dysfunction of the whole enzyme. Defects in UMP synthase is associated with hypochromic anemia. In mammals, this bifunctional enzyme UMPS converts orotic acid into uridine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
UMP Synthase
The enzyme Uridine monophosphate synthase (, UMPS) (orotate phosphoribosyl transferase and orotidine-5'-decarboxylase) catalyses the formation of uridine monophosphate (UMP), an energy-carrying molecule in many important biosynthetic pathways. In humans, the gene that codes for this enzyme is located on the long arm of chromosome 3 (3q13). Structure and function This bifunctional enzyme has two main domains, an orotate phosphoribosyltransferase (OPRTase, ) subunit and an orotidine-5’-phosphate decarboxylase (ODCase, ) subunit. These two sites catalyze the last two steps of the de novo uridine monophosphate (UMP) biosynthetic pathway. After addition of ribose-P to orotate by OPRTase to form orotidine-5’-monophosphate (OMP), OMP is decarboxylated to form uridine monophosphate by ODCase. In microorganisms, these two domains are separate proteins, but, in multicellular eukaryotes, the two catalytic sites are expressed on a single protein, uridine monophosphate synthase. UMPS ex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uridine 5'- Monophosphate
Uridine monophosphate (UMP), also known as 5′-uridylic acid (conjugate base uridylate), is a nucleotide that is used as a monomer in RNA. It is an ester of phosphoric acid with the nucleoside uridine. UMP consists of the phosphate group, the pentose sugar ribose, and the nucleobase uracil; hence, it is a ribonucleotide monophosphate. As a substituent or radical its name takes the form of the prefix uridylyl-. The deoxy form is abbreviated dUMP. Covalent attachment of UMP (e.g. to a protein such as adenylyltransferase) is called uridylylation (or sometimes uridylation). Biosynthesis Uridine monophosphate is formed from Orotidine 5'-monophosphate (orotidylic acid) in a decarboxylation reaction catalyzed by the enzyme orotidylate decarboxylase. Uncatalyzed, the decarboxylation reaction is extremely slow (estimated to occur on average one time per 78 million years). Adequately catalyzed, the reaction takes place once per second, an increase of 1017-fold. In humans, the orotidyla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orotic Aciduria
Orotic aciduria (AKA hereditary orotic aciduria) is a disease caused by an enzyme deficiency resulting in a decreased ability to synthesize pyrimidines. It was the first described enzyme deficiency of the ''de novo'' pyrimidine synthesis pathway. Orotic aciduria is characterized by excessive excretion of orotic acid in urine because of the inability to convert orotic acid to UMP. It causes megaloblastic anemia and may be associated with mental and physical developmental delays. Signs and symptoms Patients typically present with excessive orotic acid in the urine, failure to thrive, developmental delay, and megaloblastic anemia which cannot be cured by administration of vitamin B12 or folic acid. Cause and genetics This autosomal recessive disorder is caused by a deficiency in the enzyme UMPS, a bifunctional protein that includes the enzyme activities of OPRT and ODC. In one study of three patients, UMPS activity ranged from 2-7% of normal levels. Two types of orotic acidur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleotides
Nucleotides are organic molecules consisting of a nucleoside and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both of which are essential biomolecules within all life-forms on Earth. Nucleotides are obtained in the diet and are also synthesized from common nutrients by the liver. Nucleotides are composed of three subunit molecules: a nucleobase, a five-carbon sugar (ribose or deoxyribose), and a phosphate group consisting of one to three phosphates. The four nucleobases in DNA are guanine, adenine, cytosine and thymine; in RNA, uracil is used in place of thymine. Nucleotides also play a central role in metabolism at a fundamental, cellular level. They provide chemical energy—in the form of the nucleoside triphosphates, adenosine triphosphate (ATP), guanosine triphosphate (GTP), cytidine triphosphate (CTP) and uridine triphosphate (UTP)—throughout the cell for the many cellular fun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |