|
Orbital Motion (quantum)
Quantum orbital motion involves the quantum mechanical motion of rigid particles (such as electrons) about some other mass, or about themselves. Typically, orbital motion in classical motion is characterized by orbital angular momentum (the orbital motion of the center of mass) and spin, which is the motion about the center of mass. In quantum mechanics, there are analogous forms of spin and angular momentum, however they differ fundamentally from the models of classical bodies. For example, an electron (one of the main particles of concern in quantum mechanics) exhibits very quantum mechanical behavior in its motion around the nucleus of an atom which cannot be explained by classical mechanics. Orbital Angular Momentum When quantum mechanics refers to the orbital angular momentum of an electron, it is generally referring to the spatial wave equation that represents the electron's motion around the nucleus of an atom. Electrons do not "orbit" the nucleus in the classical sense of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrons
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron's mass is approximately 1/1836 that of the proton. Quantum mechanical properties of the electron include an intrinsic angular momentum ( spin) of a half-integer value, expressed in units of the reduced Planck constant, . Being fermions, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle. Like all elementary particles, electrons exhibit properties of both particles and waves: They can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a longer de Broglie wav ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spin Operator
Spin is a conserved quantity carried by elementary particles, and thus by composite particles (hadrons) and atomic nuclei. Spin is one of two types of angular momentum in quantum mechanics, the other being ''orbital angular momentum''. The orbital angular momentum operator is the quantum-mechanical counterpart to the classical angular momentum of orbital revolution and appears when there is periodic structure to its wavefunction as the angle varies. For photons, spin is the quantum-mechanical counterpart of the polarization of light; for electrons, the spin has no classical counterpart. The existence of electron spin angular momentum is inferred from experiments, such as the Stern–Gerlach experiment, in which silver atoms were observed to possess two possible discrete angular momenta despite having no orbital angular momentum. The existence of the electron spin can also be inferred theoretically from the spin–statistics theorem and from the Pauli exclusion principle—and vi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lorentz-violating Neutrino Oscillations
Lorentz-violating neutrino oscillation refers to the quantum phenomenon of neutrino oscillations described in a framework that allows the breakdown of Lorentz invariance. Today, neutrino oscillation or change of one type of neutrino into another is an experimentally verified fact; however, the details of the underlying theory responsible for these processes remain an open issue and an active field of study. The conventional model of neutrino oscillations assumes that neutrinos are massive, which provides a successful description of a wide variety of experiments; however, there are a few oscillation signals that cannot be accommodated within this model, which motivates the study of other descriptions. In a theory with Lorentz violation, neutrinos can oscillate with and without masses and many other novel effects described below appear. The generalization of the theory by incorporating Lorentz violation has shown to provide alternative scenarios to explain all the established experim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron's mass is approximately 1/1836 that of the proton. Quantum mechanical properties of the electron include an intrinsic angular momentum ( spin) of a half-integer value, expressed in units of the reduced Planck constant, . Being fermions, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle. Like all elementary particles, electrons exhibit properties of both particles and waves: They can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a longer de Broglie wavele ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
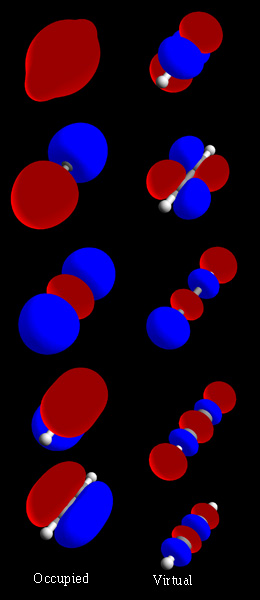
Molecular Orbital
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The terms ''atomic orbital'' and ''molecular orbital'' were introduced by Robert S. Mulliken in 1932 to mean ''one-electron orbital wave functions''. At an elementary level, they are used to describe the ''region'' of space in which a function has a significant amplitude. In an isolated atom, the orbital electrons' location is determined by functions called atomic orbitals. When multiple atoms combine chemically into a molecule, the electrons' locations are determined by the molecule as a whole, so the atomic orbitals combine to form molecular orbitals. The electrons from the constituent atoms occupy the molecular orbitals. Mathematically, molecular orbitals are an approximate solution to the Schrödin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Orbital
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term ''atomic orbital'' may also refer to the physical region or space where the electron can be calculated to be present, as predicted by the particular mathematical form of the orbital. Each orbital in an atom is characterized by a set of values of the three quantum numbers , , and , which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component (magnetic quantum number). Alternative to the magnetic quantum number, the orbitals are often labeled by the associated harmonic polynomials (e.g., ''xy'', ). Each such orbital can be occupied by a maximum of two electrons, each with its own projection of spin m_s. The simple names s orbital, p orb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantization (physics)
In physics, quantization (in British English quantisation) is the systematic transition procedure from a classical understanding of physical phenomena to a newer understanding known as quantum mechanics. It is a procedure for constructing quantum mechanics from classical mechanics. A generalization involving infinite degrees of freedom is field quantization, as in the "quantization of the electromagnetic field", referring to photons as field " quanta" (for instance as light quanta). This procedure is basic to theories of atomic physics, chemistry, particle physics, nuclear physics, condensed matter physics, and quantum optics. Historical overview In 1901, when Max Planck was developing the distribution function of statistical mechanics to solve ultraviolet catastrophe problem, he realized that the properties of blackbody radiation can be explained by the assumption that the amount of energy must be in countable fundamental units, i.e. amount of energy is not continuous b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stationary State
A stationary state is a quantum state with all observables independent of time. It is an eigenvector of the energy operator (instead of a quantum superposition of different energies). It is also called energy eigenvector, energy eigenstate, energy eigenfunction, or energy eigenket. It is very similar to the concept of atomic orbital and molecular orbital in chemistry, with some slight differences explained below. Introduction A stationary state is called ''stationary'' because the system remains in the same state as time elapses, in every observable way. For a single-particle Hamiltonian, this means that the particle has a constant probability distribution for its position, its velocity, its spin, etc. (This is true assuming the particle's environment is also static, i.e. the Hamiltonian is unchanging in time.) The wavefunction itself is not stationary: It continually changes its overall complex phase factor, so as to form a standing wave. The oscillation frequency of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Total Angular Momentum
In quantum mechanics, the total angular momentum quantum number parametrises the total angular momentum of a given particle, by combining its orbital angular momentum and its intrinsic angular momentum (i.e., its spin). If s is the particle's spin angular momentum and ℓ its orbital angular momentum vector, the total angular momentum j is \mathbf j = \mathbf s + \boldsymbol ~. The associated quantum number is the main total angular momentum quantum number ''j''. It can take the following range of values, jumping only in integer steps: , \ell - s, \le j \le \ell + s where ''ℓ'' is the azimuthal quantum number (parameterizing the orbital angular momentum) and ''s'' is the spin quantum number (parameterizing the spin). The relation between the total angular momentum vector j and the total angular momentum quantum number ''j'' is given by the usual relation (see angular momentum quantum number) \Vert \mathbf j \Vert = \sqrt \, \hbar The vector's ''z''-projection is given b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Angular Momentum
In physics, angular momentum (rarely, moment of momentum or rotational momentum) is the rotational analog of linear momentum. It is an important physical quantity because it is a conserved quantity—the total angular momentum of a closed system remains constant. Angular momentum has both a direction and a magnitude, and both are conserved. Bicycles and motorcycles, frisbees, rifled bullets, and gyroscopes owe their useful properties to conservation of angular momentum. Conservation of angular momentum is also why hurricanes form spirals and neutron stars have high rotational rates. In general, conservation limits the possible motion of a system, but it does not uniquely determine it. The three-dimensional angular momentum for a point particle is classically represented as a pseudovector , the cross product of the particle's position vector (relative to some origin) and its momentum vector; the latter is in Newtonian mechanics. Unlike linear momentum, angular m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Magnetic Resonance
Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field (in the near field) and respond by producing an electromagnetic signal with a frequency characteristic of the magnetic field at the nucleus. This process occurs near resonance, when the oscillation frequency matches the intrinsic frequency of the nuclei, which depends on the strength of the static magnetic field, the chemical environment, and the magnetic properties of the isotope involved; in practical applications with static magnetic fields up to ca. 20 tesla, the frequency is similar to VHF and UHF television broadcasts (60–1000 MHz). NMR results from specific magnetic properties of certain atomic nuclei. Nuclear magnetic resonance spectroscopy is widely used to determine the structure of organic molecules in solution and study molecular physics and crystals as well as non-crystalline materials. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cambridge University Press
Cambridge University Press is the university press of the University of Cambridge. Granted letters patent by Henry VIII of England, King Henry VIII in 1534, it is the oldest university press A university press is an academic publishing house specializing in monographs and scholarly journals. Most are nonprofit organizations and an integral component of a large research university. They publish work that has been reviewed by schola ... in the world. It is also the King's Printer. Cambridge University Press is a department of the University of Cambridge and is both an academic and educational publisher. It became part of Cambridge University Press & Assessment, following a merger with Cambridge Assessment in 2021. With a global sales presence, publishing hubs, and offices in more than 40 Country, countries, it publishes over 50,000 titles by authors from over 100 countries. Its publishing includes more than 380 academic journals, monographs, reference works, school and uni ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |