|
Oxidative Deamination
Oxidative deamination is a form of deamination that generates α-keto acids and other oxidized products from amine-containing compounds, and occurs primarily in the liver. Oxidative deamination is stereospecific, meaning it contains different stereoisomers as reactants and products; this process is either catalyzed by L or D- amino acid oxidase and L-amino acid oxidase is present only in the liver and kidney. Oxidative deamination is an important step in the catabolism of amino acids, generating a more metabolizable form of the amino acid, and also generating ammonia as a toxic byproduct. The ammonia generated in this process can then be neutralized into urea via the urea cycle. Much of the oxidative deamination occurring in cells involves the amino acid glutamate, which can be oxidatively deaminated by the enzyme glutamate dehydrogenase (GDH), using NAD or NADP as a coenzyme. This reaction generates α-ketoglutarate (α-KG) and ammonia. Glutamate can then be regenerated from α-K ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deamination
Deamination is the removal of an amino group from a molecule. Enzymes that catalyse this reaction are called deaminases. In the human body, deamination takes place primarily in the liver, however it can also occur in the kidney. In situations of excess protein intake, deamination is used to break down amino acids for energy. The amino group is removed from the amino acid and converted to ammonia. The rest of the amino acid is made up of mostly carbon and hydrogen, and is recycled or oxidized for energy. Ammonia is toxic to the human system, and enzymes convert it to urea or uric acid by addition of carbon dioxide molecules (which is not considered a deamination process) in the urea cycle, which also takes place in the liver. Urea and uric acid can safely diffuse into the blood and then be excreted in urine. Deamination reactions in DNA Cytosine Spontaneous deamination is the hydrolysis reaction of cytosine into uracil, releasing ammonia in the process. This can occur in vitro thr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coenzyme
A cofactor is a non-protein chemical compound or metallic ion that is required for an enzyme's role as a catalyst (a catalyst is a substance that increases the rate of a chemical reaction). Cofactors can be considered "helper molecules" that assist in biochemical transformations. The rates at which these happen are characterized in an area of study called enzyme kinetics. Cofactors typically differ from ligands in that they often derive their function by remaining bound. Cofactors can be divided into two types: inorganic ions and complex organic molecules called coenzymes. Coenzymes are mostly derived from vitamins and other organic essential nutrients in small amounts. (Note that some scientists limit the use of the term "cofactor" for inorganic substances; both types are included here.) Coenzymes are further divided into two types. The first is called a "prosthetic group", which consists of a coenzyme that is tightly (or even covalently) and permanently bound to a protein. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
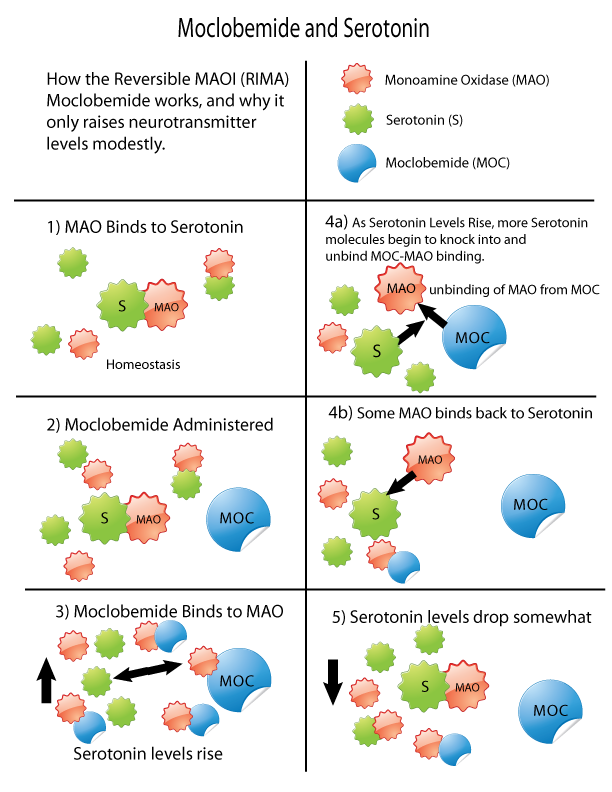
Monoamine Oxidase Inhibitor
Monoamine oxidase inhibitors (MAOIs) are a class of drugs that inhibit the activity of one or both monoamine oxidase enzymes: monoamine oxidase A (MAO-A) and monoamine oxidase B (MAO-B). They are best known as effective antidepressants, especially for treatment-resistant depression and atypical depression. They are also used to treat panic disorder, social anxiety disorder, Parkinson's disease, and several other disorders. Reversible inhibitors of monoamine oxidase A (RIMAs) are a subclass of MAOIs that selectively and reversibly inhibit the MAO-A enzyme. RIMAs are used clinically in the treatment of depression and dysthymia. Due to their reversibility, they are safer in single-drug overdose than the older, irreversible MAOIs, and weaker in increasing the monoamines important in depressive disorder. RIMAs have not gained widespread market share in the United States. Medical uses MAOIs have been found to be effective in the treatment of panic disorder with agoraphobia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epinephrine
Adrenaline, also known as epinephrine, is a hormone and medication which is involved in regulating visceral functions (e.g., respiration). It appears as a white microcrystalline granule. Adrenaline is normally produced by the adrenal glands and by a small number of neurons in the medulla oblongata. It plays an essential role in the fight-or-flight response by increasing blood flow to muscles, heart output by acting on the SA node, pupil dilation response, and blood sugar level. It does this by binding to alpha and beta receptors. It is found in many animals, including humans, and some single-celled organisms. It has also been isolated from the plant ''Scoparia dulcis'' found in Northern Vietnam. Medical uses As a medication, it is used to treat several conditions, including allergic reaction anaphylaxis, cardiac arrest, and superficial bleeding. Inhaled adrenaline may be used to improve the symptoms of croup. It may also be used for asthma when other treatments are not e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serotonin
Serotonin () or 5-hydroxytryptamine (5-HT) is a monoamine neurotransmitter. Its biological function is complex and multifaceted, modulating mood, cognition, reward, learning, memory, and numerous physiological processes such as vomiting and vasoconstriction. Approximately 90% of the serotonin that the body produces is in the intestinal tract. Biochemically, the indoleamine molecule derives from the amino acid tryptophan, via the (rate-limiting) hydroxylation of the 5 position on the ring (forming the intermediate 5-hydroxytryptophan), and then decarboxylation to produce serotonin. Serotonin is primarily found in the enteric nervous system located in the gastrointestinal tract (GI tract). However, it is also produced in the central nervous system (CNS), specifically in the raphe nuclei located in the brainstem, Merkel cells located in the skin, pulmonary neuroendocrine cells and taste receptor cells in the tongue. Additionally, serotonin is stored in blood platelets and is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoamine Neurotransmitter
Monoamine neurotransmitters are neurotransmitters and neuromodulators that contain one amino group connected to an aromaticity, aromatic ring by a two-carbon chain (such as -CH2-CH2-). Examples are dopamine, norepinephrine and serotonin. All monoamines are derived from aromatic amino acids like phenylalanine, tyrosine, and tryptophan by the action of aromatic amino acid decarboxylase enzymes. They are deactivated in the body by the enzymes known as monoamine oxidases which clip off the amine group. Monoaminergic systems, i.e., the networks of neurons that use monoamine neurotransmitters, are involved in the regulation of processes such as emotion, arousal, and certain types of memory. It has also been found that monoamine neurotransmitters play an important role in the secretion and production of neurotrophin-3 by astrocytes, a chemical which maintains neuron integrity and provides neurons with trophic support. Drugs used to increase or reduce the effect of monoamine neurotrans ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoamine Oxidase
Monoamine oxidases (MAO) () are a family of enzymes that catalyze the oxidation of monoamines, employing oxygen to clip off their amine group. They are found bound to the outer membrane of mitochondria in most cell types of the body. The first such enzyme was discovered in 1928 by Mary Bernheim in the liver and was named tyramine oxidase. The MAOs belong to the protein family of flavin-containing amine oxidoreductases. MAOs are important in the breakdown of monoamines ingested in food, and also serve to inactivate monoamine neurotransmitters. Because of the latter, they are involved in a number of psychiatric and neurological diseases, some of which can be treated with monoamine oxidase inhibitors (MAOIs) which block the action of MAOs. Subtypes and tissue distribution In humans there are two types of MAO: MAO-A and MAO-B. * Both are found in neurons and astroglia. * Outside the central nervous system: ** MAO-A is also found in the liver, pulmonary vascular endothelium, gas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transaminase
Transaminases or aminotransferases are enzymes that catalyze a transamination reaction between an amino acid and an α-keto acid. They are important in the synthesis of amino acids, which form proteins. Function and mechanism An amino acid contains an amine (NH2) group. A keto acid contains a keto (=O) group. In transamination, the NH2 group on one molecule is exchanged with the =O group on the other molecule. The amino acid becomes a keto acid, and the keto acid becomes an amino acid. Most transaminases are protein enzymes. However, some transamination activities of the ribosome have been found to be catalyzed by ribozymes (RNA enzymes). Examples being the hammerhead ribozyme, the VS ribozyme and the hairpin ribozyme. Transaminases require the coenzyme pyridoxal phosphate, which is converted into pyridoxamine in the first half-reaction, when an amino acid is converted into a keto acid. Enzyme-bound pyridoxamine in turn reacts with pyruvate, oxaloacetate, or alpha-ketoglut ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nicotinamide Adenine Dinucleotide Phosphate
Nicotinamide adenine dinucleotide phosphate, abbreviated NADP or, in older notation, TPN (triphosphopyridine nucleotide), is a Cofactor (biochemistry), cofactor used in anabolic reactions, such as the Calvin cycle and lipid and nucleic acid syntheses, which require NADPH as a reducing agent ('hydrogen source'). It is used by all forms of cellular life. NADPH is the redox, reduced form of NADP. NADP differs from NAD+, NAD by the presence of an additional phosphate group on the 2' position of the ribose ring that carries the adenine Moiety (chemistry), moiety. This extra phosphate is added by NAD+ kinase, NAD+ kinase and removed by NADP+ phosphatase. Biosynthesis NADP In general, NADP+ is synthesized before NADPH is. Such a reaction usually starts with NAD+, NAD+ from either the de-novo or the salvage pathway, with NAD+ kinase, NAD+ kinase adding the extra phosphate group. ADP-ribosyl cyclase allows for synthesis from nicotinamide in the salvage pathway, and NADP+ phosphatase ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Keto Acid
In organic chemistry, keto acids or ketoacids (also called oxo acids or oxoacids) are organic compounds that contain a carboxylic acid group () and a ketone group ().Franz Dietrich Klingler, Wolfgang Ebertz "Oxocarboxylic Acids" in Ullmann's Encyclopedia of Industrial Chemistry 2005, Wiley-VCH, Weinheim. In several cases, the keto group is hydrated. The alpha-keto acids are especially important in biology as they are involved in the Krebs citric acid cycle and in glycolysis. Common types of keto acids include: *Alpha-keto acids, alpha-ketoacids, or 2-oxoacids have the keto group adjacent to the carboxylic acid. They often arise by oxidative deamination of amino acids, and reciprocally, they are precursors to the same. Alpha-keto acids possesses extensive chemistry as acylation agents. Furthermore, alpha-keto acids such as phenylpyruvic acid are endogenous sources for carbon monoxide (as a gasotransmitter) and pharmaceutical prodrug scaffold. Important representatives: ** ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nicotinamide Adenine Dinucleotide
Nicotinamide adenine dinucleotide (NAD) is a coenzyme central to metabolism. Found in all living cells, NAD is called a dinucleotide because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine nucleobase and the other nicotinamide. NAD exists in two forms: an oxidized and reduced form, abbreviated as NAD and NADH (H for hydrogen), respectively. In metabolism, nicotinamide adenine dinucleotide is involved in redox reactions, carrying electrons from one reaction to another. The cofactor is, therefore, found in two forms in cells: NAD is an oxidizing agent – it accepts electrons from other molecules and becomes reduced. This reaction, also with H+, forms NADH, which can then be used as a reducing agent to donate electrons. These electron transfer reactions are the main function of NAD. However, it is also used in other cellular processes, most notably as a substrate of enzymes in adding or removing chemical groups to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |