|
Otamixaban
Otamixaban ( INN) is an experimental injectable anticoagulant direct factor Xa inhibitor that was investigated for the treatment for acute coronary syndrome Acute coronary syndrome (ACS) is a syndrome (a set of signs and symptoms) due to decreased blood flow in the coronary arteries such that part of the heart muscle is unable to function properly or dies. The most common symptom is centrally loca .... In 2013, Sanofi announced that it had ended development of the drug candidate after poor performance in a Phase III clinical trial. References Direct Xa inhibitors {{blood-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anticoagulant
Anticoagulants, commonly known as blood thinners, are chemical substances that prevent or reduce coagulation of blood, prolonging the clotting time. Some of them occur naturally in blood-eating animals such as leeches and mosquitoes, where they help keep the bite area unclotted long enough for the animal to obtain some blood. As a class of medications, anticoagulants are used in therapy for thrombotic disorders. Oral anticoagulants (OACs) are taken by many people in pill or tablet form, and various intravenous anticoagulant dosage forms are used in hospitals. Some anticoagulants are used in medical equipment, such as sample tubes, blood transfusion bags, heart–lung machines, and dialysis equipment. One of the first anticoagulants, warfarin, was initially approved as a rodenticide. Anticoagulants are closely related to antiplatelet drugs and thrombolytic drugs by manipulating the various pathways of blood coagulation. Specifically, antiplatelet drugs inhibit platelet agg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Direct Factor Xa Inhibitor
Direct may refer to: Mathematics * Directed set, in order theory * Direct limit of (pre), sheaves * Direct sum of modules, a construction in abstract algebra which combines several vector spaces Computing * Direct access (other), a method of accessing data in a database * Direct connect (other), various methods of telecommunications and computer networking * Direct memory access, access to memory by hardware subsystems independently of the CPU Entertainment * ''Direct'' (Tower of Power album) * ''Direct'' (Vangelis album) * ''Direct'' (EP), by The 77s Other uses * Nintendo Direct, an online presentation frequently held by Nintendo * Mars Direct, a proposal for a crewed mission to Mars * DIRECT, a proposed space shuttle-derived launch vehicle * DirectX, a proprietary dynamic media platform * Direct current, a direct flow of electricity * Direct examination, the in-trial questioning of a witness by the party who has called him or her to testify See ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acute Coronary Syndrome
Acute coronary syndrome (ACS) is a syndrome (a set of signs and symptoms) due to decreased blood flow in the coronary arteries such that part of the heart muscle is unable to function properly or dies. The most common symptom is centrally located pressure-like chest pain, often radiating to the left shoulder or angle of the jaw, and associated with nausea and sweating. Many people with acute coronary syndromes present with symptoms other than chest pain, particularly women, older people, and people with diabetes mellitus. Acute coronary syndrome is subdivided in three scenarios depending on the duration of symptoms, the presence of ECG changes and blood test results: ST elevation myocardial infarction (STEMI, 30%), non-ST elevation myocardial infarction (NSTEMI, 25%), or unstable angina (38%). Generally, when symptoms occur for less than 30 minutes, it is unstable angina. When symptoms are prolonged for more than 30 minutes, the diagnosis is acute myocardial infarction. ACS ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sanofi
Sanofi S.A. is a French multinational pharmaceutical and healthcare company headquartered in Paris, France. Originally, the corporation was established in 1973 and merged with Synthélabo in 1999 to form Sanofi-Synthélabo. In 2004, Sanofi-Synthélabo merged with Aventis and renamed to Sanofi-Aventis, which were each the product of several previous mergers. It changed its name back to Sanofi in May 2011. The company is a component of the Euro Stoxx 50 stock market index. Sanofi engages in the research and development, manufacturing and marketing of pharmaceutical drugs principally in the prescription market, but the firm also develops over-the-counter medication. The corporation covers seven major therapeutic areas: cardiovascular, central nervous system, diabetes, internal medicine, oncology, thrombosis and vaccines (it is the world's largest producer of the latter through its subsidiary Sanofi Pasteur). History Sanofi-Synthélabo Sanofi was founded in 1973 as a subsidia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
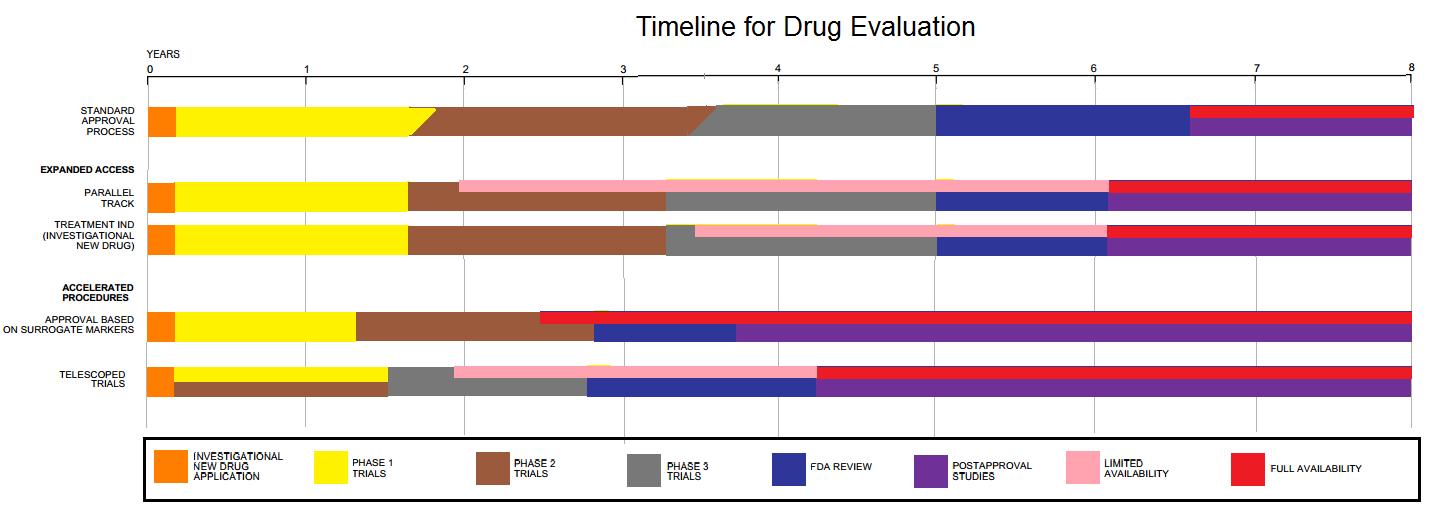
Drug Development
Drug development is the process of bringing a new pharmaceutical drug to the market once a lead compound has been identified through the process of drug discovery. It includes preclinical research on microorganisms and animals, filing for regulatory status, such as via the United States Food and Drug Administration for an investigational new drug to initiate clinical trials on humans, and may include the step of obtaining regulatory approval with a new drug application to market the drug. The entire process – from concept through preclinical testing in the laboratory to clinical trial development, including Phase I–III trials – to approved vaccine or drug typically takes more than a decade. New chemical entity development Broadly, the process of drug development can be divided into preclinical and clinical work. Pre-clinical New chemical entities (NCEs, also known as new molecular entities or NMEs) are compounds that emerge from the process of drug discovery. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase III Clinical Trial
The phases of clinical research are the stages in which scientists conduct experiments with a health intervention to obtain sufficient evidence for a process considered effective as a medical treatment. For drug development, the clinical phases start with testing for safety in a few human subjects, then expand to many study participants (potentially tens of thousands) to determine if the treatment is effective. Clinical research is conducted on drug candidates, vaccine candidates, new medical devices, and new diagnostic assays. Summary Clinical trials testing potential medical products are commonly classified into four phases. The drug development process will normally proceed through all four phases over many years. If the drug successfully passes through Phases I, II, and III, it will usually be approved by the national regulatory authority for use in the general population. Phase IV trials are 'post-marketing' or 'surveillance' studies conducted to monitor safety over sever ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |