|
Osmotic Dehydration
Osmotic dehydration is an operation used for the partial removal of water from plant tissues by immersion in a hypertonic (osmotic) solution. Sugar or salt solutions are used to reduce the moisture content of foods before actual drying process. This technique is used to give the product quality improvement over conventional drying process. Mild heat treatment after osmotic dehydration favours colour and flavour retention resulting in the product having superior organoleptic characteristics. It also increases resistance to heat treatment, prevents enzymatic browning and inhibits activities of polyphenol oxidase. The process is economical. Osmotic dehydration depends on: * Temperature of osmotic solution. * Concentration of the osmotic solution. * Osmotic agent used. * Process duration. * Geometry of food material. Process Water removal is based on the natural and non-destructive phenomenon of osmosis across cell membranes. The driving force for the diffusion of water from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tonicity
In chemical biology, tonicity is a measure of the effective osmotic pressure gradient; the water potential of two solutions separated by a partially-permeable cell membrane. Tonicity depends on the relative concentration of selective membrane-impermeable solutes across a cell membrane which determine the direction and extent of osmotic flux. It is commonly used when describing the swelling-versus-shrinking response of cells immersed in an external solution. Unlike osmotic pressure, tonicity is influenced only by solutes that cannot cross the membrane, as only these exert an effective osmotic pressure. Solutes able to freely cross the membrane do not affect tonicity because they will always equilibrate with equal concentrations on both sides of the membrane without net solvent movement. It is also a factor affecting imbibition. There are three classifications of tonicity that one solution can have relative to another: ''hypertonic'', ''hypotonic'', and ''isotonic''.A hypotonic s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
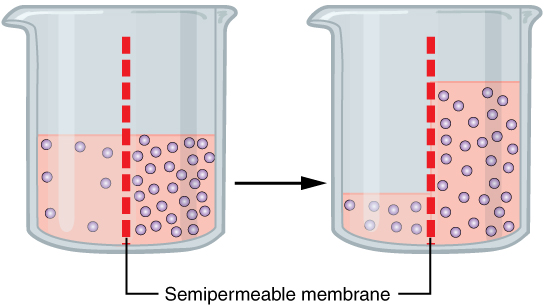
Osmosis
Osmosis (, ) is the spontaneous net movement or diffusion of solvent molecules through a selectively-permeable membrane from a region of high water potential (region of lower solute concentration) to a region of low water potential (region of higher solute concentration), in the direction that tends to equalize the solute concentrations on the two sides. It may also be used to describe a physical process in which any solvent moves across a selectively permeable membrane (permeable to the solvent, but not the solute) separating two solutions of different concentrations. Osmosis can be made to do work. Osmotic pressure is defined as the external pressure required to be applied so that there is no net movement of solvent across the membrane. Osmotic pressure is a colligative property, meaning that the osmotic pressure depends on the molar concentration of the solute but not on its identity. Osmosis is a vital process in biological systems, as biological membranes are semiperm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salting (food)
Salting is the preservation of food with dry edible salt."Historical Origins of Food Preservation." Accessed June 2011. |
Food Drying
Food drying is a method of food preservation in which food is dried (dehydrated or desiccated). Drying inhibits the growth of bacteria, yeasts, and mold through the removal of water. Dehydration has been used widely for this purpose since ancient times; the earliest known practice is 12,000 B.C. by inhabitants of the modern Middle East and Asia regions."Historical Origins of Food Preservation". Accessed June 2011. Water is traditionally removed through by using methods such as air drying, sun drying, smoking or wind drying, although today electric |
Heat Treatment
Heat treating (or heat treatment) is a group of industrial, thermal and metalworking processes used to alter the physical, and sometimes chemical, properties of a material. The most common application is metallurgical. Heat treatments are also used in the manufacture of many other materials, such as glass. Heat treatment involves the use of heating or chilling, normally to extreme temperatures, to achieve the desired result such as hardening or softening of a material. Heat treatment techniques include annealing, case hardening, precipitation strengthening, tempering, carburizing, normalizing and quenching. Although the term ''heat treatment'' applies only to processes where the heating and cooling are done for the specific purpose of altering properties intentionally, heating and cooling often occur incidentally during other manufacturing processes such as hot forming or welding. Physical processes Metallic materials consist of a microstructure of small crystals called "gr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organoleptic
Organoleptic properties are the aspects of food, water or other substances that create an individual experience via the senses—including taste, sight, smell, and touch. USDA uses In traditional U.S. Department of Agriculture meat and poultry inspections, inspectors perform various organoleptic procedures to detect disease or contamination. Such techniques are part of the effort to detect invisible foodborne pathogens that cause food poisoning. Organoleptic tests are sometimes conducted to determine if food or pharmaceutical products can transfer tastes or odors to the materials and components they are packaged in. Shelf life studies often use taste, sight, and smell (in addition to food chemistry and toxicology tests) to determine whether a food product is safe to consume. Organoleptic analyses are, occasionally, still used when the protocol for a certain sample does not have a high enough sample throughput to meet the demand. In this case, organoleptic analyses serve as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzymatic Browning
Browning is the process of food turning brown due to the chemical reactions that take place within. The process of browning is one of the chemical reactions that take place in food chemistry and represents an interesting research topic regarding health, nutrition, and food technology. Though there are many different ways food chemically changes over time, browning in particular falls into two main categories: enzymatic versus non-enzymatic browning processes. Browning has many important implications on the food industry relating to nutrition, technology, and economic cost. Researchers are especially interested in studying the control (inhibition) of browning and the different methods that can be employed to maximize this inhibition and ultimately prolong the shelf life of food. Enzymatic browning Enzymatic browning is one of the most important reactions that takes place in most fruits and vegetables as well as in seafood. These processes affect the taste, color, and value ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyphenol Oxidase
Polyphenol oxidase (PPO; also polyphenol oxidase i, chloroplastic), an enzyme involved in fruit browning, is a tetramer that contains four atoms of copper per molecule. PPO may accept monophenols and/or ''o''-diphenols as substrates. The enzyme works by catalyzing the ''o''-hydroxylation of monophenol molecules in which the benzene ring contains a single hydroxyl substituent to ''o''-diphenols (phenol molecules containing two hydroxyl substituents at the 1, 2 positions, with no carbon between). It can also further catalyse the oxidation of ''o''-diphenols to produce ''o''-quinones. PPO catalyses the rapid polymerization of ''o''-quinones to produce black, brown or red pigments (polyphenols) that cause fruit browning. The amino acid tyrosine contains a single phenolic ring that may be oxidised by the action of PPOs to form ''o''-quinone. Hence, PPOs may also be referred to as tyrosinases. Common foods producing the enzyme include mushrooms (''Agaricus bisporus''), appl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cell Membrane
The cell membrane (also known as the plasma membrane (PM) or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of all cells from the outside environment (the extracellular space). The cell membrane consists of a lipid bilayer, made up of two layers of phospholipids with cholesterols (a lipid component) interspersed between them, maintaining appropriate membrane fluidity at various temperatures. The membrane also contains membrane proteins, including integral proteins that span the membrane and serve as membrane transporters, and peripheral proteins that loosely attach to the outer (peripheral) side of the cell membrane, acting as enzymes to facilitate interaction with the cell's environment. Glycolipids embedded in the outer lipid layer serve a similar purpose. The cell membrane controls the movement of substances in and out of cells and organelles, being selectively permeable to ions a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solutes
In chemistry, a solution is a special type of homogeneous mixture composed of two or more substances. In such a mixture, a solute is a substance dissolved in another substance, known as a solvent. If the attractive forces between the solvent and solute particles are greater than the attractive forces holding the solute particles together, the solvent particles pull the solute particles apart and surround them. These surrounded solute particles then move away from the solid solute and out into the solution. The mixing process of a solution happens at a scale where the effects of chemical polarity are involved, resulting in interactions that are specific to solvation. The solution usually has the state of the solvent when the solvent is the larger fraction of the mixture, as is commonly the case. One important parameter of a solution is the concentration, which is a measure of the amount of solute in a given amount of solution or solvent. The term "aqueous solution" is used when ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Acid
An organic acid is an organic compound with acidic properties. The most common organic acids are the carboxylic acids, whose acidity is associated with their carboxyl group –COOH. Sulfonic acids, containing the group –SO2OH, are relatively stronger acids. Alcohols, with –OH, can act as acids but they are usually very weak. The relative stability of the conjugate base of the acid determines its acidity. Other groups can lsoconfer acidity, usually weakly: the thiol group –SH, the enol group, and the phenol group. In biological systems, organic compounds containing these groups are generally referred to as organic acids. A few common examples include: * lactic acid * acetic acid * formic acid * citric acid * oxalic acid * uric acid * malic acid * tartaric acid Characteristics In general, organic acids are weak acids and do not dissociate completely in water, whereas the strong mineral acids do. Lower molecular mass organic acids such as formic and lactic ac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reducing Sugar
A reducing sugar is any sugar that is capable of acting as a reducing agent. In an alkaline solution, a reducing sugar forms some aldehyde or ketone, which allows it to act as a reducing agent, for example in Benedict's reagent. In such a reaction, the sugar becomes a carboxylic acid. All monosaccharides are reducing sugars, along with some disaccharides, some oligosaccharides, and some polysaccharides. The monosaccharides can be divided into two groups: the aldoses, which have an aldehyde group, and the ketoses, which have a ketone group. Ketoses must first tautomerize to aldoses before they can act as reducing sugars. The common dietary monosaccharides galactose, glucose and fructose are all reducing sugars. Disaccharides are formed from two monosaccharides and can be classified as either reducing or nonreducing. Nonreducing disaccharides like sucrose and trehalose have glycosidic bonds between their anomeric carbons and thus cannot convert to an open-chain form with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



.png)